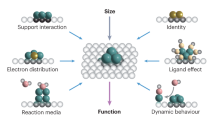
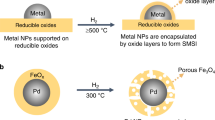
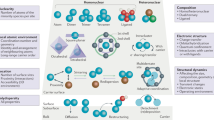
Abstract
Metal nanoparticles stabilized on a support material catalyse many major industrial reactions. Metal-support interactions in these nanomaterials can have a substantial influence on the catalysis, making metal-support interaction modulation one of the few tools able to enhance catalytic performance. This topic has received much attention in recent years, however, a systematic rationalization of the field is lacking due to the great diversity in catalysts, reactions and modification strategies. In this review, we cover and categorize the recent progress in metal-support interaction tuning strategies to enhance catalytic performance for various reactions. Furthermore, we quantify the productivity enhancements resulting from metal-support interaction control that have been achieved in C1 chemistry in recent years. Our analysis shows that up to fifteen-fold productivity enhancement has been achieved, and that metal-support interaction is most impactful for metal nanoparticles smaller than four nanometres. These findings demonstrate the importance of metal-support interaction to improve performance in catalysis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Anderson, J.A. & García, M.F. Supported Metals in Catalysis: Catalytic Science Series Vol. 5 (Imperial College Press, 2005).
Roldan Cuenya, B. Synthesis and catalytic properties of metal nanoparticles: size, shape, support, composition, and oxidation state effects. Thin Solid Films 518, 3127–3150 (2010).
Zečević, J., Vanbutsele, G., de Jong, K. P. & Martens, J. A. Nanoscale intimacy in bifunctional catalysts for selective conversion of hydrocarbons. Nature 528, 245–248 (2015).
Arnal, P. M., Comotti, M. & Schüth, F. High-temperature-stable catalysts by hollow sphere encapsulation. Angew. Chem. Int. Ed. 45, 8224–8227 (2006).
Pacchioni, G. & Freund, H.-J. Controlling the charge state of supported nanoparticles in catalysis: lessons from model systems. Chem. Soc. Rev. 47, 8474–8502 (2018).
Farmer, Ja & Campbell, C. T. Ceria maintains smaller metal catalyst particles by strong metal-support bonding. Science 329, 933–936 (2010).
Ahmadi, M., Mistry, H. & Roldan Cuenya, B. Tailoring the catalytic properties of metal nanoparticles via support interactions. J. Phys. Chem. Lett. 7, 3519–3533 (2016).
Ro, I., Resasco, J. & Christopher, P. Approaches for understanding and controlling interfacial effects in oxide-supported metal catalysts. ACS Catal. 8, 7368–7387 (2018).
Pan, C.-J. et al. Tuning/exploiting strong metal-support interaction (SMSI) in heterogeneous catalysis. J. Taiwan Inst. Chem. Eng. 74, 154–186 (2017).
Fujiwara, K., Okuyama, K. & Pratsinis, S. E. Metal–support interactions in catalysts for environmental remediation. Environ. Sci. Nano 4, 2076–2092 (2017).
Chen, M. S. & Goodman, D. W. The structure of catalytically active gold on titania. Science 306, 252–255 (2004).
Luches, P. et al. Nature of Ag islands and nanoparticles on the CeO2(111) surface. J. Phys. Chem. C. 116, 1122–1132 (2011).
Pacchioni, G. Electronic interactions and charge transfers of metal atoms and clusters on oxide surfaces. Phys. Chem. Chem. Phys. 15, 1737–1757 (2013).
Puigdollers, A. R., Schlexer, P., Tosoni, S. & Pacchioni, G. Increasing oxide reducibility: the role of metal/oxide interfaces in the formation of oxygen vacancies. ACS Catal. 7, 6493–6513 (2017).
Molina, L. M. & Hammer, B. Some recent theoretical advances in the understanding of the catalytic activity of Au. Appl. Catal. A Gen. 291, 21–31 (2005).
Zhang, B. & Qin, Y. Interface tailoring of heterogeneous catalysts by atomic layer deposition. ACS Catal. 8, 10064–10081 (2018).
Lin, X. et al. Characterizing low-coordinated atoms at the periphery of MgO-supported Au islands using scanning tunneling microscopy and electronic structure calculations. Phys. Rev. B. 81, 6–9 (2010).
Hammer, B. Special sites at noble and late transition metal catalysts. Top. Catal. 37, 3–16 (2006).
Farnesi Camellone, M., Negreiros Ribeiro, F., Szabová, L., Tateyama, Y. & Fabris, S. Catalytic proton dynamics at the water/solid interface of ceria-supported Pt clusters. J. Am. Chem. Soc. 138, 11560–11567 (2016).
Toebes, M. L. et al. Support effects in hydrogenation of cinnamaldehyde over carbon nanofiber-supported platinum catalysts: kinetic modelling. Chem. Eng. Sci. 60, 5682–5695 (2005).
Davis, S. E., Ide, M. S. & Davis, R. J. Selective oxidation of alcohols and aldehydes over supported metal nanoparticles. Green. Chem. 15, 17–45 (2013).
Conner, W. C. & Falconer, J. L. Spillover in heterogeneous catalysis. Chem. Rev. 95, 759–788 (1995).
Prins, R. Hydrogen spillover: facts and fiction. Chem. Rev. 112, 2714–2738 (2012).
Takakusagi, S., Fukui, K. I., Tero, R., Asakura, K. & Iwasawa, Y. First direct visualization of spillover species emitted from Pt nanoparticles. Langmuir 26, 16392–16396 (2010).
Karim, W. et al. Catalyst support effects on hydrogen spillover. Nature 541, 68–71 (2017).
Roldan Cuenya, B. Metal nanoparticle catalysts beginning to shape-up. Acc. Chem. Res. 46, 1682–1691 (2012).
Frenkel, A. I. et al. Correlating particle size and shape of supported Ru/γ-Al2O3 catalysts with NH3 decomposition activity. J. Am. Chem. Soc. 131, 12230–12239 (2009).
Henry, C. R. Morphology of supported nanoparticles. Prog. Surf. Sci. 80, 92–116 (2005).
Hansen, P. L. et al. Atom-resolved imaging of dynamic shape changes in supported copper nanocrystals. Science 295, 2053–2055 (2002).
Hemmingson, S. L. & Campbell, C. T. Trends in adhesion energies of metal nanoparticles on oxide surfaces: understanding support effects in catalysis and nanotechnology. ACS Nano 11, 1196–1203 (2017).
Ahmadi, M., Behafarid, F. & Roldan Cuenya, B. Size-dependent adhesion energy of shape-selected Pd and Pt nanoparticles. Nanoscale 8, 11635–11641 (2016).
Tanase, M. et al. Interfacial bonding stabilizes rhodium and rhodium oxide nanoparticles on layered Nb oxide and Ta oxide dupports. J. Am. Chem. Soc. 136, 5687–5696 (2014).
Duan, M. et al. Reconstruction of supported metal nanoparticles in reaction conditions. Angew. Chem. Int. Ed. 57, 6464–6469 (2018).
Lin, Y. et al. Adhesion and atomic structures of gold on ceria nanostructures: the role of surface structure and oxidation state of ceria rupports. Nano Lett. 15, 5375–5381 (2015).
Pingel, T. N., Jørgensen, M., Yankovich, A. B., Grönbeck, H. & Olsson, E. Influence of atomic site-specific strain on catalytic activity of supported nanoparticles. Nat. Commun. 9, 2722 (2018). Platinum nanoparticles anchored to γ-Al 2O 3 or CeO 2 show different levels of strain, which the authors quantify and they calculate the optimal Pt NP strain pattern for CO oxidation.
Shibata, N. et al. Interface structures of gold nanoparticles on TiO2 (110). Phys. Rev. Lett. 102, 136105 (2009).
Bartholomew, C. H. Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 212, 17–60 (2001).
van Deelen, T. W., Nijhuis, J. J., Krans, N. A., Zečević, J. & de Jong, K. P. Preparation of cobalt nanocrystals supported on metal oxides to study particle growth in Fischer−Tropsch catalysts. ACS Catal. 8, 10581–10589 (2018).
Penner, S. & Armbrüster, M. Formation of intermetallic compounds by reactive metal– support interaction: a frequently encountered phenomenon in catalysis. ChemCatChem 7, 374–392 (2015).
Furukawa, S. & Komatsu, T. Intermetallic compounds: promising inorganic materials for well-structured and electronically modified reaction environments for efficient catalysis. ACS Catal. 7, 735–765 (2017).
Zafeiratos, S., Piccinin, S. & Teschner, D. Alloys in catalysis: phase separation and surface segregation phenomena in response to the reactive environment. Catal. Sci. Technol. 2, 1787–1801 (2012).
Singh, A. K. & Xu, Q. Synergistic catalysis over bimetallic alloy nanoparticles. ChemCatChem 5, 652–676 (2013).
Ferrando, R., Jellinek, J. & Johnston, R. L. Nanoalloys: From theory to applications of alloy clusters and nanoparticles. Chem. Rev. 108, 845–910 (2008).
Tauster, S. J., Fung, S. C. & Garten, R. L. Strong metal-support interactions: group 8 noble metals supported on titanium dioxide. J. Am. Chem. Soc. 100, 170–175 (1978).
Tauster, S. J., Fung, S. C., Baker, R. T. & Horsley, J. A. Strong interactions in supported-metal catalysts. Science 211, 1121–1125 (1981).
Hernández-Cristóbal, O., Arenas-Alatorre, J., Díaz, G., Bahena, D. & J. Yacamán, M. High resolution HAADF characterization of Ir/TiO2 catalyst reduced at 500 °C: intensity profile analysis. J. Phys. Chem. C. 119, 11672–11678 (2015).
Willinger, M. G. et al. A case of strong metal–support interactions: combining advanced microscopy and model systems to elucidate the atomic structure of interfaces. Angew. Chem. Int. Ed. 53, 5998–6001 (2014).
Zhang, S. et al. Dynamical observation and detailed description of catalysts under strong metal−support interaction. Nano Lett. 16, 4528–4534 (2016).
Chen, M. S. & Goodman, D. W. Interaction of Au with titania: the role of reduced Ti. Top. Catal. 44, 41–47 (2007).
Saavedra, J., Pursell, C. J. & Chandler, B. D. CO oxidation kinetics over Au/TiO2 and Au/Al2O3 catalysts: evidence for a common water-assisted mechanism. J. Am. Chem. Soc. 140, 3712–3723 (2018).
Wang, Y., Widmann, D. & Behm, R. J. Influence of TiO2 bulk defects on CO adsorption and CO oxidation on Au/TiO2: electronic metal−support interactions (EMSIs) in supported Au catalysts. ACS Catal. 7, 2339–2345 (2017).
Wang, Y. et al. The role of electronic metal-support interactions and its temperature dependence: CO adsorption and CO oxidation on Au/TiO2 catalysts in the presence of TiO2 bulk defects. J. Catal. 354, 46–60 (2017).
Kumar, G. et al. Evaluating differences in the active-site electronics of supported Au nanoparticle catalysts using Hammett and DFT studies. Nat. Chem. 10, 268–274 (2018). The catalytic performance of gold nanoparticles on various supports is compared using a model reaction to obtain information on the role of charge transfer between metal and support.
Song, H. et al. Visible-light-mediated methane activation for steam methane reforming under mild conditions: a case study of Rh/TiO2 catalysts. ACS Catal. 8, 7556–7565 (2018).
Sakamoto, H. et al. Hot-electron-induced highly efficient O2 activation by Pt nanoparticles supported on Ta2O5 driven by visible light. J. Am. Chem. Soc. 137, 9324–9332 (2015).
Jackson, C. et al. Electronic metal-support interaction enhanced oxygen reduction activity and stability of boron carbide supported platinum. Nat. Commun. 8, 15802 (2017). Platinum nanoparticles supported on boron carbide outperform commercial Pt/C in terms of activity and stability, which is ascribed to an electronic metal-support interaction.
Irvine, J. T. S. et al. Evolution of the electrochemical interface in high-temperature fuel cells and electrolysers. Nat. Energy 1, 1–13 (2016).
Neagu, D., Tsekouras, G., Miller, D. N., Ménard, H. & Irvine, J. T. S. In situ growth of nanoparticles through control of non-stoichiometry. Nat. Chem. 5, 916–923 (2013).
Neagu, D. et al. Nano-socketed nickel particles with enhanced coking resistance grown in situ by redox exsolution. Nat. Commun. 6, 8120 (2015).
Gao, Y., Wang, J., Lyu, Y.-Q., Lam, K. & Ciucci, F. In situ growth of Pt3Ni nanoparticles on an A-site deficient perovskite with enhanced activity for the oxygen reduction reaction. J. Mater. Chem. A 5, 6399–6404 (2017).
Huang, X., Zhao, G., Wang, G. & Irvine, J. T. S. Synthesis and applications of nanoporous perovskite metal oxides. Chem. Sci. 9, 3623–3637 (2018).
Murata, K. et al. The metal-support interaction concerning the particle size Effect of Pd/Al2O3 on Methane Combustion. Angew. Chem. Int. Ed. 56, 15993–15997 (2017).
Bertella, F., Concepción, P. & Martínez, A. TiO2 polymorph dependent SMSI effect in Co-Ru/TiO2 catalysts and its relevance to Fischer–Tropsch synthesis. Catal. Today 289, 181–191 (2017).
Yu, L. et al. Influence of the crystal structure of titanium oxide on the catalytic activity of Rh/TiO2 in steam reforming of propane at low temperature. Chem. Eur. J. 24, 8742–8746 (2018).
Bertella, F., Concepción, P. & Martínez, A. The impact of support surface area on the SMSI decoration effect and catalytic performance for Fischer-Tropsch synthesis of Co-Ru/TiO2-anatase catalysts. Catal. Today 296, 170–180 (2017).
Abdel-Mageed, A. M. et al. Selective CO methanation on Ru/TiO2 Catalysts: role and influence of metal-support interactions. ACS Catal. 5, 6753–6763 (2015).
Yoon, S. et al. Specific metal-support interactions between nanoparticle layers for catalysts with enhanced methanol oxidation activity. ACS Catal. 8, 5391–5398 (2018).
Lin, B. et al. Effect of ceria morphology on the catalytic activity of Co/CeO2 catalyst for ammonia synthesis. Catal. Commun. 101, 15–19 (2017).
Ma, Z., Zhao, S., Pei, X., Xiong, X. & Hu, B. New insights into the support morphology-dependent ammonia synthesis activity of Ru/CeO2. catalysts. Catal. Sci. Technol. 7, 191–199 (2017).
Ha, H., Yoon, S., An, K. & Kim, H. Y. Catalytic CO oxidation over Au nanoparticles supported on CeO2 nanocrystals: effect of the Au–CeO2 interface. ACS Catal. 8, 11491–11501 (2018).
Liu, M.-H., Chen, Y.-W., Lin, T.-S. & Mou, C.-Y. Defective mesocrystal ZnO-supported gold catalysts: facilitating CO oxidation via vacancy defects in ZnO. ACS Catal. 8, 6862–6869 (2018).
Zhu, W. et al. Taming interfacial electronic properties of platinum nanoparticles on vacancy-abundant boron nitride nanosheets for enhanced catalysis. Nat. Commun. 8, 15291 (2017).
Yan, X. et al. Nickel@Siloxene catalytic nanosheets for high-performance CO2 methanation. Nat. Commun. 10, 2608 (2019).
Zhang, F. et al. Tailoring the oxidation activity of Pt nanoclusters via encapsulation. ACS Catal. 5, 1381–1385 (2015).
Li, Z. et al. Reactive metal–support interactions at moderate temperature in two-dimensional niobium-carbide-supported platinum catalysts. Nat. Catal. 1, 349–355 (2018). A Pt-Nb surface alloy with enhanced water–gas shift kinetics is generated under reaction conditions from a two-dimensional metal carbide using the concept of reactive metal-support interactions.
Li, Z. et al. Two-dimensional transition metal carbides as supports for tuning the chemistry of catalytic nanoparticles. Nat. Commun. 9, 5258 (2018).
Shi, L., Li, Z., Dao, T. D., Nagao, T. & Yang, Y. A synergistic interaction between isolated Au nanoparticles and oxygen vacancies in an amorphous black TiO2 nanoporous film: toward enhanced photoelectrochemical water splitting. J. Mater. Chem. A 6, 12978–12984 (2018).
He, L., Weniger, F., Neumann, H. & Beller, M. Synthesis, characterization, and application of metal nanoparticles supported on nitrogen-doped carbon: catalysis beyond electrochemistry. Angew. Chem. Int. Ed. 55, 12582–12594 (2016).
Shi, R. et al. Nitrogen-doped graphene supported copper catalysts for methanol oxidative carbonylation: enhancement of catalytic activity and stability by nitrogen species. Carbon 130, 185–195 (2018).
Ning, X. et al. Electron transfer dependent catalysis of Pt on N-doped carbon nanotubes: effects of synthesis method on metal-support interaction. J. Catal. 348, 100–109 (2017).
Walczak, R. et al. Template- and metal-free synthesis of nitrogen-rich nanoporous “noble” varbon materials by direct pyrolysis of a preorganized hexaazatriphenylene precursor. Angew. Chem. Int. Ed. 57, 10765–10770 (2018).
Antonietti, M. & Oschatz, M. The concept of “noble, heteroatom-doped carbons,” their directed synthesis by electronic band control of carbonization, and applications in catalysis and energy materials. Adv. Mater. 30, 1706836 (2018).
Qin, Q., Heil, T., Antonietti, M. & Oschatz, M. Single-site gold catalysts on hierarchical N-doped porous noble carbon for enhanced electrochemical reduction of nitrogen. Small Methods 2, 1800202 (2018). Gold nanoparticles are positively charged on a C 2N noble carbon support, making them active for ammonia synthesis.
Theofanidis, S. A. et al. Fe-containing magnesium aluminate support for stability and carbon control during methane reforming. ACS Catal. 8, 5983–5995 (2018).
Margossian, T. et al. Molecularly tailored nickel precursor and support yield a stable methane dry reforming catalyst with superior metal utilization. J. Am. Chem. Soc. 139, 6919–6927 (2017).
Horlyck, J., Lewis, S., Amal, R. & Scott, J. The impact of La doping on dry reforming Ni-based catalysts loaded on FSP-alumina. Top. Catal. 61, 1842–1855 (2018).
Hsieh, B.-J. et al. Platinum loaded on dual-doped TiO2 as an active and durable oxygen reduction reaction catalyst. NPG Asia Mater. 9, e403 (2017).
Tran, S. B. T., Choi, H. S., Oh, S. Y., Moon, S. Y. & Park, J. Y. Iron-doped ZnO as a support for Pt-based catalysts to improve activity and stability: enhancement of metal-support interaction by the doping effect. RSC Adv. 8, 21528–21533 (2018).
Wang, F. et al. Enhanced catalytic performance of Ir catalysts supported on ceria-based solid solutions for methane dry reforming reaction. Catal. Today 281, 295–303 (2017).
Tabakova, T. et al. Structure-activity relationship in water-gas shift reaction over gold catalysts supported on Y-doped ceria. J. Rare Earths 37, 383–392 (2019).
Chen, P. et al. Experimental and theoretical understanding of nitrogen-doping-induced strong metal-support interactions in Pd/TiO2 catalysts for nitrobenzene hydrogenation. ACS Catal. 7, 1197–1206 (2017).
Schumann, J. et al. Promoting strong metal support interaction: doping ZnO for enhanced activity of Cu/ZnO:M (M = Al, Ga, Mg) catalysts. ACS Catal. 5, 3260–3270 (2015).
Chernyak, S. A. et al. Effect of Co crystallinity on Co/CNT catalytic activity in CO/CO2 hydrogenation and CO disproportionation. Appl. Surf. Sci. 372, 100–107 (2016).
Chernyak, S. A. et al. Co catalysts supported on oxidized CNTs: evolution of structure during preparation, reduction and catalytic test in Fischer-Tropsch synthesis. Appl. Catal. A Gen. 523, 221–229 (2016).
Eschemann, T. O. et al. Effect of support surface treatment on the synthesis, structure, and performance of Co/CNT Fischer–Tropsch catalysts. J. Catal. 328, 130–138 (2015).
Honma, T. & Wayman, C. M. Epitaxial growth of evaporated cobalt films. J. Appl. Phys. 36, 2791–2798 (1965).
Rao, R. G. et al. Interfacial charge distributions in carbon-supported palladium catalysts. Nat. Commun. 8, 340 (2017). The selectivity of Pd/C catalysts in cinnamaldehyde hydrogenation is regulated by functional groups on the support surface, because of electronic charge redistribution at the Pd-C interface.
Shi, W. et al. Enhanced chemoselective hydrogenation through tuning the interaction between Pt nanoparticles and carbon supports: insights from identical location transmission electron microscopy and X‑ray photoelectron spectroscopy. ACS Catal. 6, 7844–7854 (2016).
Donoeva, B., Masoud, N. & De Jongh, P. E. Carbon support surface effects in the gold-catalyzed oxidation of 5-hydroxymethylfurfural. ACS Catal. 7, 4581–4591 (2017).
Celebi, M., Yurderi, M., Bulut, A., Kaya, M. & Zahmakiran, M. Palladium nanoparticles supported on amine-functionalized SiO2 for the catalytic hexavalent chromium reduction. Appl. Catal. B Environ. 180, 53–64 (2016).
Rodríguez-Gómez, A., Platero, F., Caballero, A. & Colón, G. Improving the direct synthesis of hydrogen peroxide from hydrogen and oxygen over Au-Pd/SBA-15 catalysts by selective functionalization. Mol. Catal. 445, 142–151 (2018).
Van Den Berg, R. et al. Support functionalization to retard ostwald ripening in copper methanol synthesis catalysts. ACS Catal. 5, 4439–4448 (2015).
Pan, Y.-X. et al. Photocatalytic CO2 reduction by carbon-coated indium-oxide nanobelts. J. Am. Chem. Soc. 139, 4123–4129 (2017).
Prieto, G. et al. Cobalt-catalyzed Fischer-Tropsch synthesis: chemical nature of the oxide support as a performance descriptor. ACS Catal. 5, 3323–3335 (2015).
Liu, L. & Corma, A. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles. Chem. Rev. 118, 4981–5079 (2018).
Lykhach, Y. et al. Counting electrons on supported nanoparticles. Nat. Mater. 15, 284–288 (2016). Charge donation per platinum atom in Pt/CeO 2 catalysts is quantified, showing that charge transfer is only effective at short range, with an optimum for nanoparticles consisting of 30–70 Pt atoms.
Guo, Y. et al. Low-temperature CO2 methanation over CeO2‑supported Ru single atoms, nanoclusters, and nanoparticles competitively tuned by strong metal-support interactions and H‑spillover effect. ACS Catal. 8, 6203–6215 (2018).
Yan, Y. et al. Ru/Al2O3 catalyzed CO2 hydrogenation: oxygen-exchange on metal-support interfaces. J. Catal. 367, 194–205 (2018).
Demiroglu, I. et al. Modelling free and oxide-supported nanoalloy catalysts: comparison of bulk-immiscible Pd–Ir and Au–Rh systems and influence of a TiO2 support. Faraday Discuss. 208, 53–66 (2018).
Piccolo, L. et al. Understanding and controlling the structure and segregation behaviour of AuRh nanocatalysts. Sci. Rep. 6, 1–8 (2016).
Konuspayeva, Z. et al. Au-Rh and Au-Pd nanocatalysts supported on rutile titania nanorods: structure and chemical stability. Phys. Chem. Chem. Phys. 17, 28112–28120 (2015).
Han, C. W. et al. Highly stable bimetallic AuIr/TiO2 catalyst: physical origins of the intrinsic high stability against sintering. Nano Lett. 15, 8141–8147 (2015).
Destro, P. et al. The crucial role of the support in the transformations of bimetallic nanoparticles and catalytic performance. ACS Catal. 8, 1031–1037 (2018).
Seemala, B., Cai, C. M., Wyman, C. E. & Christopher, P. Support induced control of surface composition in Cu-Ni/TiO2 catalysts enables high yield co-conversion of HMF and furfural to methylated furans. ACS Catal. 7, 4070–4082 (2017).
Liu, D. et al. Identifying dynamic structural changes of active sites in Pt–Ni bimetallic catalysts using multimodal approaches. ACS Catal. 8, 4120–4131 (2018).
Divins, N. J., Angurell, I., Escudero, C., Pérez-Dieste, V. & Llorca, J. Influence of the support on surface rearrangements of bimetallic nanoparticles in real catalysts. Science 346, 620–623 (2014). Rh and Pd are uniformly distributed in unsupported bimetallic nanoparticles at conditions close to ethanol steam reforming, whereas in CeO 2-supported bimetallic NP under the same conditions, Pd is enriched at the NP surface in and Rh coordinates to the support.
Gubó, R. et al. Variation of SMSI with the Au:Pd ratio of bimetallic nanoparticles on TiO2(110). Top. Catal. 61, 308–317 (2018).
Zhan, W. et al. Surfactant-assisted stabilization of Au colloids on solids for heterogeneous catalysis. Angew. Chem. Int. Ed. 56, 4494–4498 (2017).
Gao, X., Liu, H., Hidajat, K. & Kawi, S. Anti-coking Ni/SiO2 catalyst for dry reforming of methane: tole of oleylamine/oleic acid organic pair. ChemCatChem 7, 4188–4196 (2015).
Tang, H. et al. Classical strong metal–support interactions between gold nanoparticles and titanium dioxide. Sci. Adv. 3, e1700231 (2017). Coverage of gold nanoparticles by TiO 2 suboxides occurs during H 2 treatment at elevated temperature and limits accessibility of the Au surface, thereby quenching CO oxidation reactivity.
Tang, H. et al. Strong metal-support Interactions between gold nanoparticles and nonoxides. J. Am. Chem. Soc. 138, 56–59 (2016).
Tang, H. et al. Oxidative strong metal-support interactions (OMSI) of supported platinum-group metal catalysts. Chem. Sci. 9, 6679–6684 (2018).
Wang, L. et al. Strong metal-support interactions achieved by hydroxide-to-oxide support transformation for preparation of sinter-resistant gold nanoparticle catalysts. ACS Catal. 7, 7461–7465 (2017).
Hernández Mejía, C., Vogt, C., Weckhuysen, B.M. & de Jong, K.P. Stable niobia-supported nickel catalysts for the hydrogenation of carbon monoxide to hydrocarbons. Catal. Today https://doi.org/10.1016/j.cattod.2018.11.036 (2018).
Xu, M. et al. TiO2–x-modified Ni nanocatalyst with tunable metal–support interaction for water–gas shift reaction. ACS Catal. 7, 7600–7609 (2017).
Li, Y. et al. High temperature reduction dramatically promotes Pd/TiO2 catalyst for ambient formaldehyde oxidation. Appl. Catal. B Environ. 217, 560–569 (2017).
Ryabchuk, P. et al. Intermetallic nickel silicide nanocatalyst—a non-noble metal–based general hydrogenation catalyst. Sci. Adv. 4, eaat0761 (2018).
Serna, P. & Corma, A. Transforming nano metal nonselective particulates into chemoselective catalysts for hydrogenation of substituted nitrobenzenes. ACS Catal. 5, 7114–7121 (2015).
Rui, Z., Chen, L., Chen, H. & Ji, H. Strong metal-support interaction in Pt/TiO2 induced by mild HCHO and NaBH4 solution reduction and its effect on catalytic toluene combustion. Ind. Eng. Chem. Res. 53, 15879–15888 (2014).
Gänzler, A. M. et al. Tuning the structure of platinum particles on ceria in situ for enhancing the catalytic performance of exhaust gas catalysts. Angew. Chem. Int. Ed. 56, 13078–13082 (2017).
Gänzler, A. M. et al. Tuning the Pt/CeO2 Interface by in situ variation of the Pt particle size. ACS Catal. 8, 4800–4811 (2018). Various reducing agents lead to different degrees of platinum nanoparticle growth and ceria reduction in Pt/CeO 2-Al 2O 3, which strongly influences the catalytic CO oxidation activity.
Freakley, S. J. et al. Palladium-tin catalysts for the direct synthesis of H2O2 with high selectivity. Science 351, 965–968 (2016).
Hernández Mejía, C., van Deelen, T. W. & de Jong, K. P. Activity enhancement of cobalt catalysts by tuning metal-support interactions. Nat. Commun. 9, 4459 (2018). Reduction-oxidation-reduction pretreatments are applied to mitigate cobalt nanoparticle coverage by TiO 2 or Nb 2O 5 suboxides and lead to twice the exposed cobalt surface and activity in the Fischer-Tropsch synthesis.
Zhan, W. et al. A sacrificial coating strategy toward enhancement of metal-support interaction for ultrastable Au nanocatalysts. J. Am. Chem. Soc. 138, 16130–16139 (2016).
Phaahlamohlaka, T. N. et al. A sinter resistant Co Fischer-Tropsch catalyst promoted with Ru and supported on titania encapsulated by mesoporous silica. Appl. Catal. A Gen. 552, 129–137 (2018).
Li, H. et al. Evidence of the encapsulation model for strong metal−support interaction under oxidized conditions: a case study on TiOx/Pt(111) for CO oxidation by in situ wide spectral range infrared reflection adsorption spectroscopy. ACS Catal. 8, 10156–10163 (2018).
Weng, Z. & Zaera, F. Sub-monolayer control of mixed-oxide support composition in catalysts via atomic layer deposition: selective hydrogenation of cinnamaldehyde promoted by (SiO2-ALD)-Pt/Al2O3. ACS Catal. 8, 8513–8524 (2018).
Ro, I. et al. Role of the Cu-ZrO2 interfacial sites for conversion of ethanol to ethyl acetate and synthesis of methanol from CO2 and H2. ACS Catal. 6, 7040–7050 (2016).
Moon, S. Y., Naik, B., Jung, C.-H., Qadir, K. & Park, J. Y. Tailoring metal-oxide interfaces of oxide-encapsulated Pt/silica hybrid nanocatalysts with enhanced thermal stability. Catal. Today 265, 245–253 (2016).
Yang, N. & Bent, S. F. Investigation of inherent differences between oxide supports in heterogeneous catalysis in the absence of structural variations. J. Catal. 351, 49–58 (2017).
Kennedy, R. M. et al. Replication of SMSI via ALD: TiO2 overcoats increase Pt-catalyzed acrolein hydrogenation selectivity. Catal. Lett. 148, 2223–2232 (2018).
Zhang, J. et al. Wet-chemistry strong metal-support interactions in Titania supported Au catalysts. J. Am. Chem. Soc. 141, 2975–2983 (2019).
Wu, L., Li, B. & Zhao, C. Direct synthesis of hydrogen and dimethoxylmethane from methanol on copper/silica catalysts with optimal Cu+/Cu0 sites. ChemCatChem 10, 1140–1147 (2018).
Xu, C. et al. Interfacing with silica boosts the catalysis of copper. Nat. Commun. 9, 3367 (2018). A mesoporous SiO2 layer over a Cu/SiO2 catalyst increases the concentration of Cu-O-SiOx surface sites, which doubles the intrinsic activity in ester hydrogenation and increases the selectivity towards ethylene glycol from 23 % to 96 %.
Matsubu, J. C. et al. Adsorbate-mediated strong metal–support interactions in oxide-supported Rh catalysts. Nat. Chem. 9, 120–127 (2017). When adsorbates are present on the surface of rhodium nanoparticles prior to reduction treatment, an optimised support suboxide overlayer is generated, which steers the selectivity in CO 2 hydrogenation from predominantly CH 4 to CO.
Wang, X. et al. Sacrificial adsorbate strategy achieved strong metal–support interaction of stable Cu nanocatalysts. ACS Appl. Energy Mater. 1, 1408–1414 (2018).
Kleijn, S. E. F., Lai, S. C. S., Koper, M. T. M. & Unwin, P. R. Electrochemistry of nanoparticles. Angew. Chem. Int. Ed. 53, 3558–3586 (2014).
Vannice, M. A. The catalytic synthesis of hydrocarbons from H2/CO mixtures over the group VIII metals: V. The catalytic behavior of silica-supported metals. J. Catal. 50, 228–236 (1977).
Acknowledgements
Shell Global Solutions, the Netherlands Association for Scientific Research and Companhia Brasileira de Metalurgia e Mineração are thanked for financial support. K.P.d.J. acknowledges support from the European Research Council, EU FP7 ERC Advanced Grant no. 338846.
Author information
Authors and Affiliations
Contributions
T.W.v.D. and C.H.M. contributed equally. All authors were involved in literature survey, structuring and analysis of the data and writing of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Table 1 and Supplementary References
Rights and permissions
About this article
Cite this article
van Deelen, T.W., Hernández Mejía, C. & de Jong, K.P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat Catal 2, 955–970 (2019). https://doi.org/10.1038/s41929-019-0364-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-019-0364-x
This article is cited by
-
Ultra-stable and highly reactive colloidal gold nanoparticle catalysts protected using multi-dentate metal oxide nanoclusters
Nature Communications (2024)
-
Strategies to improve hydrogen activation on gold catalysts
Nature Reviews Chemistry (2024)
-
Hybrid oxide coatings generate stable Cu catalysts for CO2 electroreduction
Nature Materials (2024)
-
Nanoparticle proximity controls selectivity in benzaldehyde hydrogenation
Nature Catalysis (2024)
-
Advances of graphdiyne-supported metal catalysts in thermocatalytic reactions
Nano Research (2024)