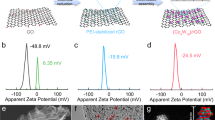
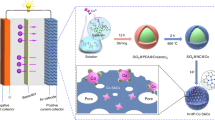
Abstract
Li–O2 batteries have received considerable attention owing to their high theoretical gravimetric energy densities. However, the sluggish kinetic barrier between gaseous O2 and solid products leads to severe polarized overpotenial. Besides, the gas-open cell architecture and cumbrous O2 storage accessories bring additional burdens on practical application. Here, by pre-embedding Li2O nanoparticles into an iridium–graphene catalytic host, we confine the O2-free reversible Li2O/Li2O2 interconversion within a sealed cell environment. After rationally controlling the depth of charge, the O2/superoxo-free charge capacity can be extended to 400 mAh g–1 (based on the entire cathodic loading mass), with only 0.12 V round-trip overpotential. Ultrastable rechargeability can be achieved for over 2,000 cycles with 99.5% coulombic efficiency. Moreover, matched with a silicon anode, the full-cell output gravimetric energy density can reach nearly 600 Wh kg–1 (based on the loading mass of both electrodes). This work shows that reversible oxide–peroxide conversion can be utilized for the development of high-energy-density sealed battery technologies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.
References
Bruce, P. G., Freunberger, S. A., Hardwick, L. J. & Tarascon, J.-M. Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 11, 19–29 (2012).
Schmuch, R. et al. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 3, 267–278 (2018).
Lu, J. et al. Aprotic and aqueous Li–O2 batteries. Chem. Rev. 114, 5611–5640 (2014).
Luntz, A. C. & McCloskey, B. D. Nonaqueous Li–air batteries: a status report. Chem. Rev. 114, 11721–11750 (2014).
Lu, Y. C. et al. Lithium–oxygen batteries: bridging mechanistic understanding and battery performance. Energy Environ. Sci. 6, 750–768 (2013).
Lim, H.-D. et al. Reaction chemistry in rechargeable Li–O2 batteries. Chem. Soc. Rev. 46, 2873–2888 (2017).
Liu, T. et al. Cycling Li–O2 batteries via LiOH formation and decomposition. Science 350, 530–533 (2015).
Lu, J. et al. A lithium–oxygen battery based on lithium superoxide. Nature 529, 377–382 (2016).
Xia, C., Kwok, C. Y. & Nazar, L. F. A high-energy-density lithium–oxygen battery based on a reversible four-electron conversion to lithium oxide. Science 361, 777 (2018).
Li, Y. & Lu, J. Metal–air batteries: will they be future electrochemical energy storage of choice? ACS Energy Lett. 2, 1370–1377 (2017).
Freunberger, S. A. True performance metrics in beyond-intercalation batteries. Nat. Energy 2, 17091 (2017).
Zhu, Z. et al. Anion-redox nanolithia cathodes for Li-ion batteries. Nat. Energy 1, 16111 (2016).
Okuoka, S.-i et al. A new sealed lithium-peroxide battery with a Co-doped Li2O cathode in a superconcentrated lithium bis(fluorosulfonyl)amide electrolyte. Sci. Rep. 4, 5684 (2014).
Kobayashi, H. et al. Improved performance of Co-doped Li2O cathodes for lithium-peroxide batteries using LiCoO2 as a dopant source. J. Power Sources 306, 567–572 (2016).
Kobayashi, H. et al. Cathode performance of Co-doped Li2O with specific capacity (400 mAh/g) enhanced by vinylene carbonate. J. Electrochem. Soc. 164, A750–A753 (2017).
Mahne, N. et al. Singlet oxygen generation as a major cause for parasitic reactions during cycling of aprotic lithium–oxygen batteries. Nat. Energy 2, 17036 (2017).
Freunberger, S. A. et al. Reactions in the rechargeable lithium–O2 battery with alkyl carbonate electrolytes. J. Am. Chem. Soc. 133, 8040–8047 (2011).
Wang, Y. et al. Mechanistic insights into catalyst-assisted nonaqueous oxygen evolution reaction in lithium–oxygen batteries. J. Phys. Chem. C. 120, 6459–6466 (2016).
Wang, Y. & Lu, Y.-C., Isotopic labeling reveals active reaction interfaces for electrochemical oxidation of lithium peroxide. Angew. Chem. Int. Ed. 58, https://doi.org/10.1002/ange.201901350 (2019).
Pi, Y. et al. Ultrathin laminar Ir superstructure as highly efficient oxygen evolution electrocatalyst in broad pH range. Nano Lett. 16, 4424–4430 (2016).
Girishkumar, G. et al. Lithium–air battery: promise and challenges. J. Phys. Chem. Lett. 1, 2193–2203 (2010).
Johnson, L. et al. The role of LiO2 solubility in O2 reduction in aprotic solvents and its consequences for Li–O2 batteries. Nat. Chem. 7, 1091–1099 (2015).
Qiao, Y. et al. From O2 − to HO2:− reducing by-products and overpotential in Li–O2 batteries by water addition. Angew. Chem. Int. Ed. 56, 4960–4964 (2017).
Chen, Y. et al. Li–O2 battery with a dimethylformamide electrolyte. J. Am. Chem. Soc. 134, 7952–7957 (2012).
Laoire, C. O. et al. Influence of nonaqueous solvents on the electrochemistry of oxygen in the rechargeable lithium-air battery. J. Phys. Chem. C. 114, 9178–9186 (2010).
Viswanathan, V. et al. Electrical conductivity in Li2O2 and its role in determining capacity limitations in non-aqueous Li–O2 batteries. J. Chem. Phys. 135, 214704–214710 (2011).
McCloskey, B. D. et al. Limitations in rechargeability of Li–O2 batteries and possible origins. J. Phys. Chem. Lett. 3, 3043–3047 (2012).
Yao, K. P. C. et al. Solid-state activation of Li2O2 oxidation kinetics and implications for Li–O2 batteries. Energy Environ. Sci. 8, 2417–2426 (2015).
Ohzuku, T. & Ueda, A. Solid-state redox reactions of LiCoO2 (R3m) for 4 volt secondary lithium cells. J. Electrochem. Soc. 141, 2972–2977 (1994).
Zhang, T. & Zhou, H. A reversible long-life lithium–air battery in ambient air. Nat. Commun 4, 1817 (2013).
Li, F. J. et al. Performance-improved Li–O2 battery with Ru nanoparticles supported on binder-free multi-walled carbon nanotube paper as cathode. Energy Environ. Sci. 7, 1648–1652 (2014).
Suo, L. et al. Fluorine-donating electrolytes enable highly reversible 5-V-class Li metal batteries. Proc. Natl Acad. Sci. 115, 1156–1161 (2018).
Asadi, M. et al. A lithium–oxygen battery with a long cycle life in an air-like atmosphere. Nature 555, 502 (2018).
Gao, X. et al. A rechargeable lithium–oxygen battery with dual mediators stabilizing the carbon cathode. Nat. Energy 2, 17118 (2017).
Pi, Y. et al. General formation of monodisperse IrM (M = Ni, Co, Fe) bimetallic nanoclusters as bifunctional electrocatalysts for acidic overall water splitting. Adv. Funct. Mater. 27, 1700886 (2017).
Zhang, L. et al. A coordinatively cross-linked polymeric network as a functional binder for high-performance silicon submicro-particle anodes in lithium-ion batteries. J. Mater. Chem. A 2, 19036–19045 (2014).
Frens, G. Controlled nucleation for regulation of particle-size in monodisperse gold suspensions. Nat. Phys. Sci. 241, 20–22 (1973).
Li, J. F. et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 464, 392–395 (2010).
Hy, S. et al. Direct in situ observation of Li2O evolution on li-rich high-capacity cathode material, Li[NixLi(1–2x)/3Mn(2–x)/3]O2 (0 ≤ x ≤0.5). J. Am. Chem. Soc. 136, 999–1007 (2014).
Qiao, Y. et al. Li–CO2 electrochemistry: a new strategy for CO2 fixation and energy storage. Joule 1, 359–370 (2017).
Qiao, Y. et al. MOF-based separator in an Li–O2 battery: an effective strategy to restrain the shuttling of dual redox mediators. ACS Energy Lett. 3, 463–468 (2018).
McCloskey, B. D. et al. Solvents’ critical role in nonaqueous lithium-oxygen battery electrochemistry. J. Phys. Chem. Lett. 2, 1161–1166 (2011).
Beyer, H. et al. Antimony doped tin oxide–synthesis, characterization and application as cathode material in Li–O2 cells: implications on the prospect of carbon-free cathodes for rechargeable lithium–air batteries. J. Electrochem. Soc. 164, A1026–A1036 (2017).
Schwenke, K. U. et al. The influence of water and protons on Li2O2 crystal growth in aprotic Li–O2 cells. J. Electrochem. Soc. 162, A573–A584 (2015).
Kwak, W.-J. et al. Synergistic integration of soluble catalysts with carbon-free electrodes for Li–O2 batteries. ACS Catal. 7, 8192–8199 (2017).
Schafzahl, B. et al. Quantifying total superoxide, peroxide, and carbonaceous compounds in metal–O2 batteries and the solid electrolyte interphase. ACS Energy Lett. 3, 170–176 (2018).
Eisenberg, G. Colorimetric determination of hydrogen peroxide. Ind. Eng. Chem. Anal. Ed. 15, 327–328 (1943).
Satterfield, C. N. & Bonnell, A. H. Interferences in titanium sulfate method for hydrogen peroxide. Anal. Chem. 27, 1174–1175 (1955).
Li, F. J. et al. The water catalysis at oxygen cathodes of lithium–oxygen cells. Nat. Common. 6, 8843 (2015).
Aetukuri, N. B. et al. Solvating additives drive solution-mediated electrochemistry and enhance toroid growth in non-aqueous Li–O2 batteries. Nat. Chem. 7, 50–56 (2015).
Acknowledgements
This work was partially supported by the National Basic Research Programme of China (grant no. 2016YFB0100203) and NSF of China (grant no. 21633003, U1801251). Financial support from the Advanced Low Carbon Technology Research and Development Programme, specially promoting research for innovative next-generation batteries (SPRING), from the Japan Science and Technology Agency is acknowledged. H.D. acknowledges scholarships from the China Scholarship Council. We thank Y. Sun (NIMS, Japan), Z. Chang (AIST, Japan) and X. Mu (Nanjing University, China) for their help in DEMS characterization, schematic cartoon production and general discussion.
Author information
Authors and Affiliations
Contributions
Y.Q. and H.Z. contributed to the design of the research and performed the experimental data analysis. Y.Q. conducted the electrochemical and spectroscopic characterizations. K.J. performed the synthesis and assessment of the Ir–rGO cathodic substrate. H.D. performed the fabrication of both the Li2O-based cathode plate and related full-cell systems. All authors co-wrote the manuscript. H.Z. supervised the work. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information
Supplementary Discussion, Supplementary Figs. 1–25, Supplementary Tables 1–3 and Supplementary References.
Rights and permissions
About this article
Cite this article
Qiao, Y., Jiang, K., Deng, H. et al. A high-energy-density and long-life lithium-ion battery via reversible oxide–peroxide conversion. Nat Catal 2, 1035–1044 (2019). https://doi.org/10.1038/s41929-019-0362-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-019-0362-z
This article is cited by
-
In situ Raman, FTIR, and XRD spectroscopic studies in fuel cells and rechargeable batteries
Nano Research (2023)
-
Advances in electrolyte safety and stability of ion batteries under extreme conditions
Nano Research (2023)
-
Porous dual carbon framework coated silicon nanoparticles for high-performance lithium-ion batteries
Journal of Materials Science: Materials in Electronics (2023)
-
Threshold potentials for fast kinetics during mediated redox catalysis of insulators in Li–O2 and Li–S batteries
Nature Catalysis (2022)
-
Pushing the limit of 3d transition metal-based layered oxides that use both cation and anion redox for energy storage
Nature Reviews Materials (2022)