Abstract
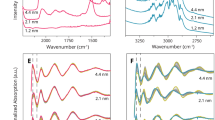
The development of improved catalysts requires insights into the relationship between catalytic activity and catalyst structure, including the underlying reaction mechanism. Here, we demonstrate a unique set of catalyst extrudate sensors that allow for the simultaneous detection of local temperature by luminescence thermometry, and of surface species by shell-isolated nanoparticle-enhanced Raman spectroscopy. This sensing approach was applied to the characterization of direct conversion of syngas into hydrocarbons and C2+ oxygenates over supported Rh and RhFe catalysts. Luminescence thermometry demonstrated a mismatch between the set temperature and the local catalyst temperature, with variations up to 40 °C. Furthermore, by investigating the surface species on varying extrudate and catalyst compositions, we identified tilted carbonyl species on the Rh/SiO2 interface that are probable precursors for the hydrogen-assisted CO dissociation. The implementation of extrudate catalyst sensors as a characterization tool provides a unique approach towards the further understanding of the relevant parameters in catalysis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.
References
Ertl, G. Reactions at Solid Surfaces (John Wiley & Sons, 2009).
Dumesic, J. A., Huber, G. W. & Boudar, M. in Handbook of Heterogeneous Catalysis (eds Ertl, G. et al.) 1–48 (Wiley-VCH, 2008).
Somorjai, G. A. & Li, Y. Introduction to Surface Chemistry and Catalysis (John Wiley & Sons, 2010).
Bañares, M. A. Operando spectroscopy: the knowledge bridge to assessing structure-performance relationships in catalyst nanoparticles. Adv. Mater. 23, 5293–5301 (2011).
Buurmans, I. L. C. & Weckhuysen, B. M. Heterogeneities of individual catalyst particles in space and time as monitored by spectroscopy. Nat. Chem. 4, 873–886 (2012).
Weckhuysen, B. M. Determining the active site in a catalytic process: operando spectroscopy is more than a buzzword. Phys. Chem. Chem. Phys. 5, 4351–43560 (2003).
Li, J. F. et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 464, 392–395 (2010).
Ding, S.-Y. et al. Nanostructure-based plasmon-enhanced Raman spectroscopy for surface analysis of materials. Nat. Rev. Mater. 1, 16021 (2016).
Li, J.-F., Zhang, Y.-J., Ding, S.-Y., Panneerselvam, R. & Tian, Z.-Q. Core-shell nanoparticle-enhanced raman spectroscopy. Chem. Rev. 117, 5002–5069 (2017).
Schlücker, S. Surface-enhanced raman spectroscopy: concepts and chemical applications. Angew. Chem. Int. Ed. Engl. 53, 4756–4795 (2014).
Wang, Y. et al. In situ analysis of surface catalytic reactions using shell-isolated nanoparticle-enhanced raman spectroscopy. Anal. Chem. 91, 1675–1685 (2019).
Hartman, T. & Weckhuysen, B. M. Thermally stable TiO2- and SiO2-shell-isolated Au nanoparticles for in situ plasmon-enhanced Raman spectroscopy of hydrogenation catalysts. Chem. Eur. J. 24, 3733–3741 (2018).
Zhang, H. et al. In situ dynamic tracking of heterogeneous nanocatalytic processes by shell-isolated nanoparticle-enhanced Raman spectroscopy. Nat. Commun. 8, 15447 (2017).
Hwang, S. & Smith, R. Optimum reactor design in methanation processes with nonuniform catalysts. Chem. Eng. Commun. 196, 616–642 (2009).
Grunwaldt, J. D., Wagner, J. B. & Dunin-Borkowski, R. E. Imaging catalysts at work: a hierarchical approach from the macro- to the meso- and nano-scale. ChemCatChem 5, 62–80 (2013).
Koptyug, I. V., Khomichev, A. V., Lysova, A. A. & Sagdeev, R. Z. Spatially resolved NMR thermometry of an operating fixed-bed catalytic reactor. J. Am. Chem. Soc. 130, 10452–10453 (2008).
Kimmerle, B. et al. Visualizing a catalyst at work during the ignition of the catalytic partial oxidation of methane. J. Phys. Chem. C 113, 3037–3040 (2009).
Simeone, M., Salemme, L., Allouis, C. & Volpicelli, G. Temperature profile in a reverse flow reactor for catalytic partial oxidation of methane by fast IR imaging. AIChE J. 54, 2689–2698 (2008).
Yarulina, I., Kapteijn, F. & Gascon, J. The importance of heat effects in the methanol to hydrocarbons reaction over ZSM-5: on the role of mesoporosity on catalyst performance. Catal. Sci. Technol. 6, 5320–5325 (2016).
Jaque, D. & Vetrone, F. Luminescence nanothermometry. Nanoscale 4, 4301–4326 (2012).
Brites, C. D. S. et al. Thermometry at the nanoscale. Nanoscale 4, 4799–4829 (2012).
Geitenbeek, R. G. et al. In situ luminescence thermometry to locally measure temperature gradients during catalytic reactions. ACS Catal. 8, 2397–2401 (2018).
Vetrone, F. et al. Temperature sensing using fluorescent nanothermometers. ACS Nano 4, 3254–3258 (2010).
Li, T., Guo, C., Zhou, S., Duan, C. & Yin, M. Highly sensitive optical thermometry of Yb3+-Er3+ codoped AgLa(MoO4)2 green upconversion phosphor. J. Am. Ceram. Soc. 98, 2812–2816 (2015).
Xu, X. et al. α-NaYb(Mn)F4:Er3+/Tm3+@NaYF4 UCNPs as “band-shape” luminescent nanothermometers over a wide temperature range. ACS Appl. Mater. Interfaces 7, 20813–20819 (2015).
Geitenbeek, R. G. et al. NaYF4:Er3+,Yb3+/SiO2 core/shell upconverting nanocrystals for luminescence thermometry up to 900 K. J. Phys. Chem. C 121, 3503–3510 (2017).
Auzel, F. Upconversion and anti-Stokes processes with f and d ions in solids. Chem. Rev. 104, 139–173 (2004).
Carnall, W. T., Crosswhite, H. & Crosswhite, H. M. Energy level structure and transition probabilities of the trivalent lanthanides in LaF 3 (Argonne National Laboratory, 1978).
Svelto, O. Principles of Lasers 4th edn (Springer, 1998).
Inoue, T., Iizuka, T. & Tanabe, K. Hydrogenation of carbon dioxide and carbon monoxide over supported rhodium catalysts under 10 bar pressure. Appl. Catal. 46, 1–9 (1989).
Rumble, J. R. CRC Handbook of Chemistry and Physics 98th edn (CRC Press, 2017).
Subramanian, N. D. et al. La and/or V oxide promoted Rh/SiO2 catalysts: effect of temperature, H2/CO ratio, space velocity, and pressure on ethanol selectivity from syngas. J. Catal. 272, 204–209 (2010).
Hongmei, Y. et al. The performance of C2 oxygenates synthesis from syngas over Rh−Mn−Li−Fe SiO2 catalysts with various Rh loadings. Energy Fuels 17, 1401–1406 (2003).
Spivey, J. J. & Egbebi, A. Heterogeneous catalytic synthesis of ethanol from biomass-derived syngas. Chem. Soc. Rev. 36, 1514–1528 (2007).
Williams, C. T., Black, C. A., Weaver, M. J. & Takoudis, C. G. Adsorption and hydrogenation of carbon monoxide on polycrystalline rhodium at high gas pressures. J. Phys. Chem. B 101, 2874–2883 (1997).
Williams, C. T., Chen, K.-Y., Takoudis, C. G. & Weaver, M. J. Reduction kinetics of surface rhodium oxide by hydrogen and carbon monoxide at ambient gas pressures as probed by transient surface-enhanced raman spectroscopy. J. Phys. Chem. B 102, 4785–4794 (1998).
Arakawa, H. et al. High pressure in situ FT-IR study of CO hydrogenation over Rh/SiO2 catalyst. Chem. Lett. 14, 23–26 (1985).
Chuang, S. Role of silver promoter in carbon monoxide hydrogenation and ethylene hydroformylation over Rh/SiO2 catalysts. J. Catal. 138, 536–546 (1992).
Chuang, S. S. C. & Pien, S. I. Infrared study of the CO insertion reaction on reduced, oxidized, and sulfided Rh/SiO2 catalysts. J. Catal. 135, 618–634 (1992).
Paredes-Nunez, A. et al. CO hydrogenation on cobalt-based catalysts: tin poisoning unravels CO in hollow sites as a main surface intermediate. Angew. Chem. Int. Ed. Engl. 57, 547–550 (2018).
Chuang, S. S. C., Stevens, R. W. & Khatri, R. Mechanism of C2+ oxygenate synthesis on Rh catalysts. Top. Catal. 32, 225–232 (2005).
Tóth, M. et al. Hydrogenation of carbon dioxide on Rh, Au and Au-Rh bimetallic clusters supported on titanate nanotubes, nanowires and TiO2. Top. Catal. 55, 747–756 (2012).
Hadjiivanov, K. I. & Vayssilov, G. N. Characterization of oxide surfaces and zeolites by carbon monoxide as an IR probe molecule. Adv. Catal. 47, 307–511 (2002).
Hernández Mejía, C., van Deelen, T. W. & de Jong, K. P. Activity enhancement of cobalt catalysts by tuning metal-support interactions. Nat. Commun. 9, 4459 (2018).
Ichikawa, M. & Fukushima, T. Infrared studies of metal additive effects on CO chemisorption modes SiO2-supported Rh-Mn, -Ti, and -Fe catalysts. J. Phys. Chem. 89, 1564–1567 (1985).
Erdöhelyi, A. & Solymosi, F. Effects of the support on the adsorption and dissociation of CO and on the reactivity of surface carbon on Rh catalysts. J. Catal. 84, 446–460 (1983).
Degioanni, S. et al. Surface-enhanced Raman scattering of amorphous TiO2 thin films by gold nanostructures: revealing first layer effect with thickness variation. J. Appl. Phys. 114, 234307 (2013).
Weng, S. X., Anderson, A. & Torrie, B. H. Raman and far-infrared spectra of crystalline formaldehyde, H2CO and D2CO. J. Raman Spectrosc. 20, 789–794 (1989).
Mitchell, W. J., Xie, J., Jachimowski, T. A. & Weinberg, W. H. Carbon monoxide hydrogenation on the Ru(001) surface at low temperature using gas-phase atomic hydrogen: spectroscopic evidence for the carbonyl insertion mechanism on a transition metal surface. J. Am. Chem. Soc. 117, 2606–2617 (1995).
Xu, C. et al. Interfacing with silica boosts the catalysis of copper. Nat. Commun. 9, 3367 (2018).
Liu, Y. et al. Synthesis gas conversion over Rh-based catalysts promoted by Fe and Mn. ACS Catal. 7, 4550–4563 (2017).
Huang, A. X. et al. Atomic-scale observation of the metal-promoter interaction in Rh-based syngas upgrading catalysts. Angew. Chem. Int. Ed. Engl. 58, 8709–8713 (2019).
Ao, M., Pham, G. H., Sunarso, J., Tade, M. O. & Liu, S. Active centers of catalysts for higher alcohol synthesis from syngas: a review. ACS Catal. 8, 7025–7050 (2018).
Palomino, R. M., Magee, J. W., Llorca, J., Senanayake, S. D. & White, M. G. The effect of Fe–Rh alloying on CO hydrogenation to C2+ oxygenates. J. Catal. 329, 87–94 (2015).
Wang, J., Zhang, Q. & Wang, Y. Rh-catalyzed syngas conversion to ethanol: studies on the promoting effect of FeOx. Catal. Today 171, 257–265 (2011).
Koole, R. et al. On the incorporation mechanism of hydrophobic quantum dots in silica spheres by a reverse microemulsion method. Chem. Mater. 20, 2503–2512 (2008).
Li, Z. & Zhang, Y. An efficient and user-friendly method for the synthesis of hexagonal-phase NaYF4:Yb, Er/Tm nanocrystals with controllable shape and upconversion fluorescence. Nanotechnology 19, 345606 (2008).
Wang, F., Deng, R. & Liu, X. Preparation of core-shell NaGdF4 nanoparticles doped with luminescent lanthanide ions to be used as upconversion-based probes. Nat. Protoc. 9, 1634–1644 (2014).
Acknowledgements
This work was supported by the Netherlands Center for Multiscale Catalytic Energy Conversion, an Netherlands Organization for Scientific Research Gravitation programme funded by the Ministry of Education, Culture and Science of the government of the Netherlands. We thank the Netherlands Organization for Scientific Research and Shell Global Solutions in the framework of the Chemical Innovation Partnership Project for financial support. We gratefully acknowledge A. Meijerink (Utrecht University, UU) for his valuable input and discussions. We thank N. Krans (UU) for her contributions to the TEM-EDX measurements, I. ten Have (UU) for her contributions to the SEM measurements and S. van Vreeswijk (UU) and R. Oord (UU) for their help with the GC measurements.
Author information
Authors and Affiliations
Contributions
The manuscript was written with contributions of all authors. All authors have given approval to the final version of the manuscript. T.H., R.G.G. and B.M.W. conceived and designed the experiments. NaYF4@SiO2 NPs were prepared by R.G.G. and were used by G.T.W. to form extrudates. T.H prepared and deposited Au@SiO2 and Au@TiO2 SHINs with Rh on the extrudates. The operando experiments were performed by T.H. and R.G.G.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9, Supplementary Table 1 and Supplementary References.
Rights and permissions
About this article
Cite this article
Hartman, T., Geitenbeek, R.G., Whiting, G.T. et al. Operando monitoring of temperature and active species at the single catalyst particle level. Nat Catal 2, 986–996 (2019). https://doi.org/10.1038/s41929-019-0352-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-019-0352-1
This article is cited by
-
Transcending scales in catalysis for sustainable development
Nature Chemical Engineering (2024)
-
Abnormal thermally-stimulated dynamic organic phosphorescence
Nature Communications (2024)
-
Shell-isolated nanoparticle-enhanced Raman spectroscopy
Nature Reviews Methods Primers (2023)
-
Impact of bimetallic interface design on heat generation in plasmonic Au/Pd nanostructures studied by single-particle thermometry
Nature Communications (2023)
-
Mechanically excited thermometry in erbium ions
Science China Materials (2023)