Abstract
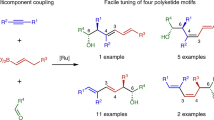
The cyclization of poly-β-carbonyl-substrates controlled by polyketide synthases intricately governs the biosynthesis of a wide range of aromatic polyketides. Analogous small-molecule-catalysed processes would conceivably induce selective cyclizations of non-canonical polycarbonyl substrates to provide products distinct from natural polyketides. Here, we report a secondary amine-catalysed twofold cyclization of non-canonical hexacarbonyl substrates, furnishing enantioenriched tetra-ortho-substituted binaphthalenes. The substrates were prepared by a fourfold ozonolysis of dicinnamyl biindenes and converted under catalyst control with high atroposelectivity. Privileged catalysts, helicenes and ligands are readily accessible from the binaphthalene products stemming from the non-canonical polyketide cyclizations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The synthetic procedures and characterization data are available in the Supplementary Information. Crystallographic data have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under nos. 1856452 (for (Ra)-4a prepared with (R)-6a, reflection shown in Table 2), 1856453 (for (Sa)-4c) and 1856454 (for (Sa)-4e) and can be obtained free of charge from the CCDC via www.ccdc.cam.ac.uk/getstructures. All other data are available from the authors upon reasonable request.
References
Hertweck, C. The biosynthetic logic of polyketide diversity. Angew. Chem. Int. Ed. 48, 4688–4716 (2009).
Crawford, J. M. & Townsend, C. A. New insights into the formation of fungal aromatic polyketides. Nat. Rev. Microbiol. 8, 879–889 (2010).
Das, A. & Koshla, C. Biosynthesis of aromatic polyketides in bacteria. Acc. Chem. Res. 42, 631–639 (2009).
Aldemir, H., Richarz, R. & Gulder, T. A. M. The biocatalytic repertoire of natural biaryl formation. Angew. Chem. Int. Ed. 53, 8286–8293 (2014).
Mazzaferro, L. S., Hüttel, W., Fries, A. & Müller, M. Cytochrome P450-catalyzed regio- and stereoselective phenol coupling of fungal natural products. J. Am. Chem. Soc. 137, 12289–12295 (2015).
Skrobo, B., Rolfes, J. D. & Deska, J. Enzymatic approaches for the preparation of optically active non-centrochiral compounds. Tetrahedron 72, 1257–1275 (2016).
Kozlowski, M. C., Morgan, B. J. & Linton, E. C. Total synthesis of chiral biaryl natural products by asymmetric biaryl coupling. Chem. Soc. Rev. 38, 3193–3207 (2009).
Bringmann, G., Gulder, T., Gulder, T. A. M. & Breuning, M. Atroposelective total synthesis of axially chiral biaryl natural products. Chem. Rev. 111, 563–639 (2011).
Liu, B., Beuerle, T., Klundt, T. & Beerhues, L. Biphenyl synthase from yeast-extract-treated cell cultures of Sorbus aucuparia. Planta 218, 492–496 (2004).
Feng, Z., Kallifidas, D. & Brady, S. F. Functional analysis of environmental DNA-derived type II polyketide synthases reveals structurally diverse secondary metabolites. Proc. Natl Acad. Sci. USA 108, 12629–12634 (2011).
Qin, Z. et al. Formicamycins, antibacterial polyketides produced by Streptomyces formicae isolated from African Tetraponera plant-ants. Chem. Sci. 8, 3218–3227 (2017).
Franke, J., Ishida, K. & Hertweck, C. Genomics-driven discovery of burkholderic acid, a noncanonical, cryptic polyketide from human pathogenic Burkholderia species. Angew. Chem. Int. Ed. 51, 11611–11615 (2012).
Harris, T. M. & Harris, C. M. Synthesis of polyketide-type aromatic natural products by biogenetically modeled routes. Tetrahedron 33, 2159–2185 (1977).
Yamaguchi, M., Okuma, T., Horiguchi, A., Ikeura, C. & Minami, T. Total synthesis of (−)-urdamycinone B through polyketide condensation. J. Org. Chem. 57, 1647–1649 (1992).
Krohn, K. Biomimetic synthesis of deca- and dodecaketide-derived quinone antibiotics. Eur. J. Org. Chem. 2002, 1351–1362 (2002).
Cookson, R., Barrett, T. N. & Barrett, A. G. M. β‑keto-dioxinones and β,δ-diketo-dioxinones in biomimetic resorcylate total synthesis. Acc. Chem. Res. 48, 628–642 (2015).
Martínez, A., Fernández, M., Estévez, J. C., Estévez, R. J. & Castedo, L. Studies on the chemistry of 2-(2-oxo-3-phenylpropyl)-benzaldehydes: novel total synthesis of 3-phenylnaphthalen-2-ols and 2-hydroxy-3-phenyl-1,4-naphthoquinones. Tetrahedron 61, 485–492 (2005).
Takikawa, H., Nishii, A. & Suzuki, K. Synthesis of β-hydroxynaphthoate derivatives from ketodioxinones via benzyne acyl-alkylation and aldol condensation cascade. Synthesis 48, 3331–3338 (2016).
Link, A. & Sparr, C. Organocatalytic atroposelective aldol condensation: synthesis of axially chiral biaryls by arene formation. Angew. Chem. Int. Ed. 53, 5458–5461 (2014).
Witzig, R. M., Lotter, D., Fäseke, V. C. & Sparr, C. Stereoselective arene‐forming aldol condensation: catalyst‐controlled synthesis of axially chiral compounds. Chem. Eur. J. 23, 12960–12966 (2017).
Link, A. & Sparr, C. Stereoselective arene formation. Chem. Soc. Rev. 47, 3804–3815 (2018).
Kočovsky, P., Vyskočil, Š. & Smrčina, M. Non-symmetrically substituted 1,1′-binaphthyls in enantioselective catalysis. Chem. Rev. 103, 3213–3246 (2003).
Guo, F., Konkol, L. C. & Thomson, R. J. Enantioselective synthesis of biphenols from 1,4-diketones by traceless central-to-axial chirality exchange. J. Am. Chem. Soc. 133, 18–20 (2011).
Jang, Y.-S., Woźniak, Ł., Pedroni, J. & Cramer, J. Access to P- and axially-chiral biaryl phosphine oxides by enantioselective CpXIrIII-catalyzed C–H arylations. Angew. Chem. Int. Ed. 57, 12901–12905 (2018).
Tanaka, K. Transition‐metal‐catalyzed enantioselective [2+2+2] cycloadditions for the synthesis of axially chiral biaryls. Chem. Asian J. 4, 508–518 (2009).
Hayashi, T., Hayashizaki, K., Kiyoi, T. & Ito, Y. Asymmetric synthesis catalyzed by chiral ferrocenylphosphine-transition-metal complexes. 6. Practical asymmetric synthesis of 1,1′-binaphthyls via asymmetric cross-coupling with a chiral [(alkoxyalkyl)ferrocenyl]monophosphine/nickel catalyst. J. Am. Chem. Soc. 110, 8153–8156 (1988).
Parmar, D., Sugiono, E., Raja, S. & Rueping, M. Complete field guide to asymmetric BINOL-phosphate derived Brønsted acid and metal catalysis: history and classification by mode of activation; Brønsted acidity, hydrogen bonding, ion pairing and metal phosphates. Chem. Rev. 114, 9047–9153 (2014).
Baldwin, J. E. & Lusch, M. J. Rules for ring closure: application to intramolecular aldol condensations in polyketonic substrates. Tetrahedron 38, 2939–2947 (1982).
Pidathala, C., Hoang, L., Vignola, N. & List, B. Direct catalytic asymmetric Enolexo aldolizations. Angew. Chem. Int. Ed. 42, 2785–2788 (2003).
Nicolet, P., Sanchez, J.-Y., Benaboura, A. & Abadie, M. J. M. Synthesis of 1,1′-biindenes and 3,3′-biindienes. Synthesis 2, 202–203 (1987).
Bringmann, G. & Jansen, J. R. Einfache Synthesen nützlicher Diketo-Bausteine für biomimetische Isochinolin- und Naphthalin-Synthesen. Liebigs Ann. Chem. 11, 2116–2125 (1985).
Kersten, L., Harms, K. & Hilt, G. Synthesis of tri‐, tetra‐ and pentacarbonyl derivatives via ozonolysis of 1,4-dienes and cyclization to polyaromatic systems. J. Org. Chem. 79, 11661–11673 (2014).
Ooi, T. & Maruoka, K. Recent advances in asymmetric phase-transfer catalysis. Angew. Chem. Int. Ed. 46, 4222–4266 (2007).
Lotter, D., Castrogiovanni, A., Neuburger, M. & Sparr, C. Catalyst-controlled stereodivergent synthesis of atropisomeric multiaxis systems. ACS Cent. Sci. 4, 656–660 (2018).
Tang, Z. et al. Novel small organic molecules for a highly enantioselective direct aldol reaction. J. Am. Chem. Soc. 125, 5262–5263 (2003).
Samanta, S., Liu, J., Dodda, R. & Zhao, C.-G. C2-symmetric bisprolinamide as a highly efficient catalyst for direct aldol reaction. Org. Lett. 7, 5321–5323 (2005).
Miura, D. & Machinami, T. Stereoselective aldol reaction in aqueous solution using prolinamido-glycosides as water-compatible organocatalyst. Mod. Res. Catal. 4, 20–27 (2015).
Tanimori, S., Naka, T. & Kirihata, M. Synthesis of a new proline‐derived organic catalyst and its evaluation for direct aldol reaction. Synth. Commun. 34, 4043–4048 (2004).
Chen, Y., Yekta, S. & Yudin, A. K. Modified BINOL ligands in asymmetric catalysis. Chem. Rev. 103, 3155–3212 (2003).
Liu, Y. & Du, H. Chiral dienes as ‘ligands’ for borane-catalyzed metal-free asymmetric hydrogenation of imines. J. Am. Chem. Soc. 135, 6810–6813 (2013).
Cao, Z. & Du, H. Development of binaphthyl-based chiral dienes for rhodium(i)-catalyzed asymmetric arylation of N,N-dimethylsulfamoyl-protected aldimines. Org. Lett. 12, 2602–2605 (2010).
Kitamura, M., Arimura, Y., Shirakawa, S. & Maruoka, K. Combinatorial approach for the design of new, simplified chiral phase-transfer catalysts with high catalytic performance for practical asymmetric synthesis of α-alkyl-α-amino acids. Tetrahedron Lett. 49, 2026–2030 (2008).
Shirakawa, S. & Maruoka, K. Recent developments in asymmetric phase-transfer reactions. Angew. Chem. Int. Ed. 52, 4312–4348 (2013).
Ravat, P. et al. Configurational stability of [5]helicenes. Org. Lett. 19, 3707–3710 (2017).
Šámal, M. et al. An ultimate stereocontrol in asymmetric synthesis of optically pure fully aromatic helicenes. J. Am. Chem. Soc. 137, 8469–8474 (2015).
Acknowledgements
We acknowledge financial support from the Swiss National Science Foundation (BSSGI0-155902/1), the University of Basel and the NCCR Molecular Systems Engineering and thank F. Zellweger and F. Bianchi for skilful technical work, T. Müntener for NMR assistance and M. Neuburger for X-ray crystallography.
Author information
Authors and Affiliations
Contributions
R.M.W. developed the substrate synthesis and identified the aldolization modes. R.M.W. and V.C.F. optimized the described synthetic method. R.M.W. investigated the scope. R.M.W. and V.C.F. synthesized the ligand, helicene and catalyst. D.H. investigated the substrate tautomerism and mechanism by NMR. C.S. conceived and supervised the project. All authors contributed to the preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, Supplementary Table 1, Supplementary Fig. 1 and Supplementary references
Compound (Ra)-4a
Crystallographic data for compound (Ra)-4a
Compound (Sa)-4c
Crystallographic data for compound (Sa)-4c
Compound (Sa)-4e
Crystallographic data for compound (Sa)-4e
Rights and permissions
About this article
Cite this article
Witzig, R.M., Fäseke, V.C., Häussinger, D. et al. Atroposelective synthesis of tetra-ortho-substituted biaryls by catalyst-controlled non-canonical polyketide cyclizations. Nat Catal 2, 925–930 (2019). https://doi.org/10.1038/s41929-019-0345-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-019-0345-0
This article is cited by
-
Enantioselective organocatalytic synthesis of axially chiral aldehyde-containing styrenes via SNAr reaction-guided dynamic kinetic resolution
Nature Communications (2023)
-
Atroposelective desymmetrization of 2-arylresorcinols via Tsuji-Trost allylation
Communications Chemistry (2023)
-
Asymmetric synthesis of binaphthyls through photocatalytic cross-coupling and organocatalytic kinetic resolution
Science China Chemistry (2022)
-
Organocatalytic dynamic kinetic resolution of N-arylindole lactams: atroposelective construction of axially chiral amino acids bearing a C-N chiral axis
Science China Chemistry (2022)
-
Organocatalytic atroposelective heterocycloaddition to access axially chiral 2-arylquinolines
Communications Chemistry (2021)