Abstract
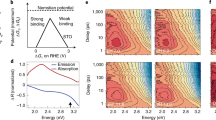
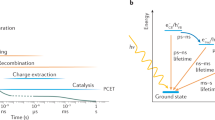
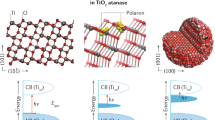
Although catalytic mechanisms on electrode surfaces have been proposed for decades, the pathways by which the product’s chemical bonds evolve from the initial charge-trapping intermediates have not been resolved. Here, we discover a reactive intermediate population with states in the middle of a semiconductor’s bandgap that reveal the dynamics of two parallel transition state pathways for their decay. After phototriggering the water oxidation reaction from the n-SrTiO3 surface, the microsecond decay of the intermediates affirms transition state theory through two distinct time constants, the primary kinetic salt and H/D kinetic isotope effects, realistic activation barrier heights and transition state theory pre-factors. Furthermore, we show that the reaction conditions can be adjusted to allow selection between the two pathways, one characterized by a labile intermediate facing the electrolyte (the oxyl), and the other by a lattice oxygen (the bridge). In summary, we experimentally isolate an important activation barrier in multi-electron transfer water oxidation and, in doing so, identify competing mechanisms for O2 evolution at surfaces.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The representative data and all of the analysis from the extended dataset that support the findings of this paper are available in the paper and the Supplementary Information. The extended dataset that supports the findings in this paper is available from the corresponding author on reasonable request.
References
Hwang, J. et al. Perovskites in catalysis and electrocatalysis. Science 358, 751–756 (2017).
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017).
Markovic, N. M. Electrocatalysis: interfacing electrochemistry. Nat. Mater. 12, 101–102 (2013).
Bockris, J. O. M. & Otagawa, T. The electrocatalysis of oxygen evolution on perovskites. J. Electrochem. Soc. 131, 290–302 (1984).
Surendranath, Y., Kanan, M. W. & Nocera, D. G. Mechanistic studies of the oxygen evolution reaction by a cobalt-phosphate catalyst at neutral pH. J. Am. Chem. Soc. 132, 16501–16509 (2010).
Yeo, B. S. & Bell, A. T. Enhanced activity of gold-supported cobalt oxide for the electrochemical evolution of oxygen. J. Am. Chem. Soc. 133, 5587–5593 (2011).
Seitz, L. et al. A highly active and stable IrOx/SrIrO3 catalyst for the oxygen evolution reaction. Science 353, 1011–1014 (2016).
Li, Y. F., Liu, Z. P., Liui, L. & Gao, W. Mechanism and activity of photocatalytic oxygen evolution on titania anatase in aqueous surroundings. J. Am. Chem. Soc. 132, 13008–13015 (2010).
Zhang, M. & Frei, H. Towards a molecular level understanding of the multi-electron catalysis of water oxidation on metal oxide surfaces. Catal. Lett. 145, 420–435 (2014).
Rossmeisl, J., Qu, Z. W., Zhu, H., Kroes, G. J. & Nørskov, J. K. Electrolysis of water on oxide surfaces. J. Electroanal. Chem. 607, 83–89 (2007).
Nørskov, J. K. et al. Universality in heterogeneous catalysis. J. Catal. 209, 275–278 (2002).
Pijpers, J. J. H., Winkler, M. T., Surendranath, Y., Buonassisi, T. & Nocera, D. G. Light-induced water oxidation at silicon electrodes functionalized with a cobalt oxygen-evolving catalyst. Proc. Natl Acad. Sci. USA 108, 10056–10061 (2011).
Blakemore, J. D., Crabtree, R. H. & Brudvig, G. W. Molecular catalysts for water oxidation. Chem. Rev. 115, 12974–13005 (2015).
Yano, J. & Yachandra, V. K. Where water is oxidized to dioxygen: structure of the photosynthetic Mn4Ca cluster from X-ray spectroscopy. Inorg. Chem. 47, 1711–1726 (2008).
Askerka, M., Brudvig, G. W. & Batista, V. S. The O2-evolving complex of photosystem II: recent insights from quantum mechanics/molecular mechanics (QM/MM), extended X-ray absorption fine structure (EXAFS), and femtosecond X-ray crystallography data. Acc. Chem. Res. 50, 41–48 (2017).
Noguchi, T. Light-induced FTIR difference spectroscopy as a powerful tool toward understanding the molecular mechanism of photosynthetic oxygen evolution. Photo. Res. 91, 59–69 (2007).
Zaharieva, I., Wichmann, J. M. & Dau, H. Thermodynamic limitations of photosynthetic water oxidation at high proton concentrations. J. Bio. Chem. 286, 18222–18228 (2011).
Zaharieva, I., Dau, H. & Haumann, M. Sequential and coupled proton and electron transfer events in the S2 → S3 transition of photosynthetic water oxidation revealed by time-resolved X-ray absorption spectroscopy. Biochemistry 55, 6996–7004 (2016).
Herlihy, D. M. et al. Detecting the oxyl radical of photocatalytic water oxidation at an n-SrTiO3/aqueous interface through its subsurface vibration. Nat. Chem. 8, 549–555 (2016).
Zandi, O. & Hamann, T. W. Determination of photoelectrochemical water oxidation intermediates on haematite electrode surfaces using operando infrared spectroscopy. Nat. Chem. 8, 778–783 (2016).
Zhang, M., de Respinis, M. & Frei, H. Time-resolved observations of water oxidation intermediates on a cobalt oxide nanoparticle catalyst. Nat. Chem. 6, 362–367 (2014).
Pham, H. H., Cheng, M.-J., Frei, H. & Wang, L.-W. Surface proton hopping and fast-kinetics pathway of water oxidation on Co3O4 (001) surface. ACS Catal. 6, 5610–5617 (2016).
Cheng, J., Vandevondele, J. & Sprik, M. Identifying trapped electronic holes at the aqueous TiO2 interface. J. Phys. Chem. C 118, 5437–5444 (2014).
Formal, F. L. et al. Rate law analysis of water oxidation on a hematite surface. J. Am. Chem. Soc. 137, 6629–6637 (2015).
Pendlebury, S. R. et al. Ultrafast charge carrier recombination and trapping in hematite photoanodes under applied bias. J. Am. Chem. Soc. 136, 9854–9857 (2014).
Chen, X. et al. The formation time of Ti–O• and Ti–O•–Ti Radicals at the n-SrTiO3/aqueous interface during photocatalytic water oxidation. J. Am. Chem. Soc. 139, 1830–1841 (2017).
Aschaffenburg, D. J., Chen, X. & Cuk, T. Faradaic oxygen evolution from SrTiO3 under nano- and femto-second pulsed light excitation. Chem. Commun. 53, 7254–7257 (2017).
Likforman, J. P., Alexandrou, A., Joffre, M. & Fejer, M. Femtosecond white-light continuum generation in a Ti:sapphire oscillator. in Advanced Solid State Lasers (eds. Bosenberg, W & Fejer, M.) Vol. 19, TS5 (Optical Society of America, 1998).
Waegele, M. M., Chen, X., Herlihy, D. M. & Cuk, T. How surface potential determines the kinetics of the first hole transfer of photocatalytic water oxidation. J. Am. Chem. Soc. 136, 10632–10639 (2014).
Yamada, Y., Yasuda, H., Tayagaki, T. & Kanemitsu, Y. Photocarrier recombination dynamics in highly excited SrTiO3 studied by transient absorption and photoluminescence spectroscopy. Appl. Phys. Lett. 95, 121112 (2009).
Higuchi, T. et al. Electronic structure in the band gap of lightly doped SrTiO3 by high-resolution X-ray absorption spectroscopy. Phys. Rev. B 61, 12860–12863 (2000).
Mitra, C., Lin, C., Robertson, J. & Demkov, A. A. Electronic structure of oxygen vacancies in SrTiO3 and LaAlO3. Phys. Rev. B 86, 155105 (2012).
Janotti, A., Varley, J. B., Choi, M. & Van de Walle, C. G. Vacancies and small polarons in SrTiO3. Phys. Rev. B 90, 085202 (2014).
Chen, H. & Umezawa, N. Hole localization, migration, and the formation of peroxide anion in perovskite SrTiO3. Phys. Rev. B 90, 035202 (2014).
Guhl, H., Miller, W. & Reuter, K. Oxygen adatoms at SrTiO3(001): a density-functional theory study. Surf. Sci. 604, 372–376 (2010).
Kafizas, A. et al. Water oxidation kinetics of accumulated holes on the surface of a TiO2 photoanode: a rate law analysis. ACS Catal. 7, 4896–4903 (2017).
Norton, A. P., Bernasek, S. L. & Bocarsly, A. B. Mechanistic aspects of the photooxidation of water at the n-titania/aqueous interface: optically induced transients as a kinetic probe. J. Phys. Chem. 92, 6009–6016 (1988).
Zhang, Y. et al. Pivotal role and regulation of proton transfer in water oxidation on hematite photoanodes. J. Am. Chem. Soc. 138, 2705–2711 (2016).
De Stefano, C., Foti, C., Giuffrè, O. & Sammartano, S. Dependence on ionic strength of protonation enthalpies of polycarboxylate anions in NaCl aqueous solution. J. Chem. Eng. Data 46, 1417–1424 (2001).
Eyring, H. The activated complex in chemical reactions. J. Chem. Phys. 3, 107–115 (1935).
Lundberg, M., Blomberg, M. R. & Siegbahn, P. E. Oxyl radical required for O–O bond formation in synthetic Mn-catalyst. Inorg. Chem. 43, 264–274 (2004).
Li, X. & Siegbahn, P. E. Alternative mechanisms for O2 release and O–O bond formation in the oxygen evolving complex of photosystem II. Phys. Chem. Chem. Phys. 17, 12168–12174 (2015).
Bronsted, J. N. Theory of the chemical reaction rate. Z. Phys. Chem. 102, 169–207 (1922).
Steinfeld, J. I., Francisco, J. S. & Hase, W. L. Chemical Kinetics and Dynamics 2nd edn (Prentice Hall, 1998).
Li, F. et al. Immobilizing Ru(bda) catalyst on a photoanode via electrochemical polymerization for light-driven water splitting. ACS Catal. 5, 3786–3790 (2015).
Oloo, W. N., Fielding, A. J. & Que, L. Rate-determining water-assisted O–O Bond Cleavage of an FeIII–OOH intermediate in a bio-inspired nonheme iron-catalyzed oxidation. J. Am. Chem. Soc. 135, 6438–6441 (2013).
Saavedra, J., Doan, H. A., Pursell, C. J., Grabow, L. C. & Chandler, B. D. The critical role of water at the gold-titania interface in catalytic CO oxidation. Science 345, 1599–1602 (2014).
Wijeratne, G. B., Day, V. W. & Jackson, T. A. O–H bond oxidation by a monomeric MnIII–OMe complex. Dalton Trans. 44, 3295–3306 (2015).p
Acknowledgements
The experimental work was supported by the Director, Office of Science, Office of Basic Energy Sciences, and by the Division of Chemical Sciences, Geosciences and Biosciences of the US Department of Energy at LBNL under contract no. DE-AC02–05CH11231. We thank H. Frei and J. Eaves for fruitful discussions.
Author information
Authors and Affiliations
Contributions
T.C. and X.C. conceived the project and T.C. wrote the manuscript with input from all authors. X.C and D.J.A constructed the transient set-up, collected transient data and prepared samples. X.C and T.C. analysed the transient data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–17, Tables 1–5, Notes 1–5 and references.
Rights and permissions
About this article
Cite this article
Chen, X., Aschaffenburg, D.J. & Cuk, T. Selecting between two transition states by which water oxidation intermediates decay on an oxide surface. Nat Catal 2, 820–827 (2019). https://doi.org/10.1038/s41929-019-0332-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-019-0332-5
This article is cited by
-
Free energy difference to create the M-OH* intermediate of the oxygen evolution reaction by time-resolved optical spectroscopy
Nature Materials (2022)
-
Understanding the Mechanism of the Oxygen Evolution Reaction with Consideration of Spin
Electrochemical Energy Reviews (2021)
-
Voltage- and time-dependent valence state transition in cobalt oxide catalysts during the oxygen evolution reaction
Nature Communications (2020)