Abstract
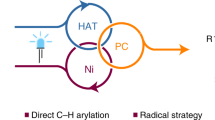
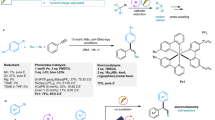
Unsaturated carbon–carbon bonds are one of the most common and important structural motifs in many organic molecules, stimulating the continuous development of general, efficient and practical strategies for their functionalization. Here, we report a one-pot difunctionalization of alkynes via a photoredox/nickel dual-catalysed three-component cross-coupling reaction under mild conditions, providing access to a series of highly important tri-substituted alkenes. Notably, in contrast to traditional methods that are based on the steric hindrance of the substrates to control the reaction selectivity, both E- and Z-isomers of tri-substituted alkenes, which are often energetically close, can be obtained by choosing an appropriate photocatalyst with a suitable triplet state energy. Beyond the immediate practicality of this transformation, this newly developed methodology might inspire the development of diverse and important one-pot functionalizations of carbon–carbon multiple bonds via photoredox and transition-metal dual-catalysed multicomponent reactions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The X-ray crystallographic coordinates for structures of 5 and 87 reported in this Article have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition numbers CCDC 1914140 (5) and 1914141 (87). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Experimental procedures and characterization of the new compounds are available in the Supplementary Information. All other data are available from the authors upon reasonable request.
References
Tietze, L. F. & Modi, A. Multicomponent domino reactions for the synthesis of biologically active natural products and drugs. Med. Res. Rev. 20, 304–322 (2000).
Masson, G., Neuville, L., Bughin, C., Fayol, A. & Zhu, J. Multicomponent syntheses of macrocycles. Top. Heterocycl. Chem. 25, 1–24 (2000).
Domling, A., Wang, W. & Wang, K. Chemistry and biology of multicomponent reactions. Chem. Rev. 112, 3083–3135 (2012).
Levi, L. & Müller, T. J. J. Multicomponent syntheses of functional chromophores. Chem. Soc. Rev. 45, 2825–2846 (2016).
Zhu, J., Wang, Q. & Wang, M.-X. (eds) Multicomponent Reactions in Organic Synthesis (Wiley, Hoboken, 2015).
Müller, T. J. J. & Deilhof, K. in Multicomponent Synthesis of Heterocycles in Multicomponent Reactions in Organic Synthesis (eds Zhu, J., Wang, Q. & Wang, M.-X.) 333–378 (Wiley, Hoboken, 2015).
Xue, F., Zhao, J., Hor, T. S. A. & Hayashi, T. Nickel-catalyzed three-component domino reactions of aryl Grignard reagents, alkynes and aryl halides producing tetrasubstituted alkenes. J. Am. Chem. Soc. 137, 3189–3192 (2015).
Satoh, T., Ogino, S., Miura, M. & Nomura, M. Synthesis of highly substituted 1,3-butadienes by palladium-catalyzed arylation of internal alkynes. Angew. Chem. Int. Ed. 43, 5063–5065 (2004).
Kischkewitz, M., Okamoto, K., Mück-Lichtenfeld, C. & Studer, A. Radical-polar crossover reactions of vinylboron ate complexes. Science 355, 936–938 (2017).
Lu, Q. et al. Dioxygen-triggered oxidative radical reaction: direct aerobic difunctionalization of terminal alkynes toward β-keto sulfones. J. Am. Chem. Soc. 135, 11481–11484 (2013).
García-Domínguez, A., Müller, S. & Nevado, C. Nickel-catalyzed intermolecular carbosulfonylation of alkynes via sulfonyl radicals. Angew. Chem. Int. Ed. 56, 9949–9952 (2017).
Li, Z., Garcia-Dominguez, A. & Nevado, C. Nickel-catalyzed stereoselective dicarbofunctionalization of alkynes. Angew. Chem. Int. Ed. 55, 6938–6941 (2016).
Li, Z., Garcia-Dominguez, A. & Nevado, C. Pd-catalyzed stereoselective carboperfluoroalkylation of alkynes. J. Am. Chem. Soc. 137, 11610–11613 (2015).
Derosa, J., Tran, V. T., Boulous, M. N., Chen, J. S. & Engle, K. M. Nickel-catalyzed β,γ-dicarbofunctionalization of alkenyl carbonyl compounds via conjunctive cross-coupling. J. Am. Chem. Soc. 139, 10657–10660 (2017).
Marzo, L., Pagire, S. K., Reiser, O. & König, B. Visible-light photocatalysis: does it make a difference in organic synthesis? Angew. Chem. Int. Ed. 57, 10034–10072 (2018).
Ye, J. H. et al. Visible-light-driven iron-promoted thiocarboxylation of styrenes and acrylates with CO2. Angew. Chem. Int. Ed. 56, 15416–15420 (2017).
Koike, T. & Akita, M. Fine design of photoredox systems for catalytic fluoromethylation of carbon–carbon multiple bonds. Acc. Chem. Res. 49, 1937–1945 (2016).
Metternich, J. B. & Gilmour, R. A bio-inspired, catalytic E → Z isomerization of activated olefins. J. Am. Chem. Soc. 137, 11254–11257 (2015).
Zuo, Z. et al. Merging photoredox with nickel catalysis: coupling of α-carboxyl sp 3-carbons with aryl halides. Science 345, 437–440 (2014).
Tellis, J. C., Primer, D. N. & Molander, G. A. Single-electron transmetalation in organoboron cross-coupling by photoredox/nickel dual catalysis. Science 345, 433–436 (2014).
Twilton, J., Zhang, P., Shaw, M. H., Evans, R. W. & MacMillan, D. W. C. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 1, 0052 (2017).
Matsui, J. K., Lang, S. B., Heitz, D. R. & Molander, G. A. Photoredox-mediated routes to radicals: the value of catalytic radical generation in synthetic methods development. ACS Catal. 7, 2563–2575 (2017).
Wang, H. et al. Markovnikov-selective radical addition of S-nucleophiles to terminal alkynes through a photoredox process. Angew. Chem. Int. Ed. 56, 595–599 (2017).
Yue, H., Zhu, C. & Rueping, M. Cross-coupling of sodium sulfinates with aryl, heteroaryl and vinyl halides by nickel/photoredox dual catalysis. Angew. Chem. Int. Ed. 57, 1371–1375 (2018).
Deng, H.-P., Fan, X.-Z., Chen, Z.-H., Xu, Q.-H. & Wu, J. Photoinduced nickel-catalyzed chemo- and regioselective hydroalkylation of internal alkynes with ether and amide α-hetero C(sp 3)–H bonds. J. Am. Chem. Soc. 139, 13579–13584 (2017).
Go, S. Y., Lee, G. S. & Hong, S. H. Highly regioselective and E/Z-selective hydroalkylation of ynone, ynoate and ynamide via photoredox mediated Ni/Ir dual catalysis. Org. Lett. 20, 4691–4694 (2018).
Singh, K., Staig, S. J. & Weaver, J. D. Facile synthesis of Z-alkenes via uphill catalysis. J. Am. Chem. Soc. 136, 5275–5278 (2014).
Fabry, D. C., Ronge, M. A. & Magnus, R. Immobilization and continuous recycling of photoredox catalysts in ionic liquids for applications in batch reactions and flow systems: catalytic alkene isomerization by using visible light. Chem. Eur. J. 21, 5350–5354 (2015).
Wei, X.-J., Boon, W., Hessel, V. & Noël, T. Visible-light photocatalytic decarboxylation of α,β-unsaturated carboxylic acids: facile access to stereoselective difluoromethylated styrenes in batch and flow. ACS Catal. 7, 7136–7140 (2017).
Wu, J., Grant, P. S., Li, X., Noble, A. & Aggarwal, V. K. Catalyst-free deaminative functionalizations of primary amines by photoinduced single-electron transfer. Angew. Chem. Int. Ed. 58, 5697–5701 (2019).
Noble, A. & MacMillan, D. W. C. Photoredox α-vinylation of α-amino acids and N-aryl amines. J. Am. Chem. Soc. 136, 11602–11605 (2014).
Gutierrez, O., Tellis, J. C., Primer, D. N., Molander, G. A. & Kozlowski, M. C. Nickel-catalyzed cross-coupling of photoredox-generated radicals: uncovering a general manifold for stereoconvergence in nickel-catalyzed cross-couplings. J. Am. Chem. Soc. 137, 4896–4899 (2015).
Oderinde, M. S. et al. Highly chemoselective iridium photoredox and nickel catalysis for the cross-coupling of primary aryl amines with aryl halides. Angew. Chem. Int. Ed. 55, 13219–13223 (2016).
Oderinde, M. S., Frenette, M., Robbins, D. W., Aquila, B. & Johannes, J. W. Photoredox mediated nickel catalyzed cross-coupling of thiols with aryl and heteroaryl iodides via thiyl radicals. J. Am. Chem. Soc. 138, 1760–1763 (2016).
Frisch, M. J. et al. Gaussian 09, revision D.01 (Gaussian, 2013).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 78, 1396 (1997).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 8, 1057–1065 (2006).
Zhao, Y. & Truhlar, D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215–241 (2008).
Marenich, A. V., Cramer, C. J. & Truhlar, D. G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396 (2009).
Kelly, C. P., Cramer, C. J. & Truhlar, D. G. SM6: a density functional theory continuum solvation model for calculating aqueous solvation free energies of neutrals, ions and solute–water clusters. J. Chem. Theory Comput. 1, 1133–1152 (2005).
Kelly, C. P., Cramer, C. J. & Truhlar, D. G. Aqueous solvation free energies of ions and ion–water clusters based on an accurate value for the absolute aqueous solvation free energy of the proton. J. Phys. Chem. B 110, 16066–16081 (2006).
Glendening, E. D., Reed, A. E., Carpenter, J. E. & Weinhold, F. NBO version 3.1 (Theoretical Chemistry Institute, 1998).
Reed, A. E., Curtiss, L. A. & Weinhold, F. Intermolecular interactions from a natural bond orbital, donor–acceptor viewpoint. Chem. Rev. 88, 899–926 (1988).
Legault, C. Y. CYLview 1.0b (Unversité de Sherbrooke, 2009); http://www.cylview.org.
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyser. J. Comput. Chem. 33, 580–592 (2012).
Acknowledgements
The authors thank D. Wöll and O. Nevskyi for assistance with measuring the emission spectra of the photocatalysts. H.Y. thanks the China Scholarship Council. C.Z., B.M., L.C. and M.R. acknowledge King Abdullah University of Science and Technology (KAUST) for support and the KAUST Supercomputing Laboratory for providing computational resources of the supercomputer Shaheen II. The research leading to these results has received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP/2007-2013) and ERC grant agreement no. 617044 (SunCatChem).
Author information
Authors and Affiliations
Contributions
C.Z., H.Y. and M.R. conceived and designed the experiments. C.Z. and H.Y. performed and analysed the experiments. I.A. conducted X-ray crystal structure analysis. C.Z., B.M. and L.C. performed the theoretical calculations. All authors discussed the results and contributed to the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, Supplementary Figs. 1–19, Supplementary Tables 1–7, Supplementary references
Supplementary Data 1
Cartesian coordinates and energies of calculated structures
Compound 5
Crystallographic data for compound 5
Compound 87
Crystallographic data for compound 87
Rights and permissions
About this article
Cite this article
Zhu, C., Yue, H., Maity, B. et al. A multicomponent synthesis of stereodefined olefins via nickel catalysis and single electron/triplet energy transfer. Nat Catal 2, 678–687 (2019). https://doi.org/10.1038/s41929-019-0311-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-019-0311-x
This article is cited by
-
Photoredox catalytic alkylarylation of alkynes with arylsulfonylacetate as bifunctional reagent
Science China Chemistry (2024)
-
Photocatalytic vinyl radical-mediated multicomponent 1,4-/1,8-carboimination across alkynes and olefins/(hetero)arenes
Science China Chemistry (2024)
-
Electrochemical chemo- and regioselective arylalkylation, dialkylation and hydro(deutero)alkylation of 1,3-enynes
Nature Synthesis (2023)
-
Direct synthesis of unprotected aryl C-glycosides by photoredox Ni-catalysed cross-coupling
Nature Synthesis (2023)
-
Radical thioesterification via nickel-catalysed sensitized electron transfer
Nature Synthesis (2023)