Abstract
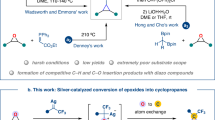
The reduction of epoxides has been recognized as an important method for the synthesis of alcohols using stoichiometric amounts of metal hydride reducing agents. However, homogeneous catalysis-enabled hydrogenation processes with molecular hydrogen remain scarce. Here, we present a general methodology for the synthesis of primary alcohols in high yields, selectively and under mild conditions, from aliphatic and aromatic epoxides. Crucial for the hydrogenation of terminal epoxides is the presence of an Fe(BF4)2.6H2O/tetraphos catalyst system. Compared to existing methods, which make use of noble metals, the presented protocol shows broad substrate scope and good functional group tolerance. The generality of this is showcased by transformation of various natural products, including steroids, terpenoids, sesquiterpenoids and drug derivatives, which give the desired alcohols in moderate to excellent yields. Mechanistic studies confirm the distinct feature of the catalyst system, which is active for Meinwald rearrangement of epoxides as well as for carbonyl hydrogenations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
CCDC 1895726 (S7), 1895727 (S9), 1895728 (S10), 1895729 (9a) and 1895730 (10′) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Further data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.
References
Weissermel, K. & Arpe, H.-J. Industrial Organic Chemistry 4th edn (Wiley-VCH, Hoboken, 2008).
Dong, G., Teo, P., Wickens, Z. K. & Grubbs, R. H. Primary alcohols from terminal olefins: formal anti-Markovnikov hydration via triple relay catalysis. Science 333, 1609–1612 (2011).
Takahashi, K., Yamashita, M., Ichihara, T., Nakano, K. & Nozaki, K. High-yielding tandem hydroformylation/hydrogenation of a terminal olefin to produce a linear alcohol using a Rh/Ru dual catalyst system. Angew. Chem. Int. Ed. 49, 4488–4490 (2010).
Torres, G. M., Frauenlob, R., Franke, R. & Börner, A. Production of alcohols via hydroformylation. Catal. Sci. Technol. 5, 34–54 (2015).
Fleischer, I. et al. From olefins to alcohols: efficient and regioselective ruthenium-catalyzed domino hydroformylation/reduction sequence. Angew. Chem. Int. Ed. 52, 2949–2953 (2013).
Yuki, Y., Takahashi, K., Tanaka, Y. & Nozaki, K. Tandem isomerization/hydroformylation/hydrogenation of internal alkenes to n-alcohols using Rh/Ru dual- or ternary-catalyst systems. J. Am. Chem. Soc. 135, 17393–17400 (2013).
Diab, L., Šmejkal, T., Geier, J. & Breit, B. Supramolecular catalyst for aldehyde hydrogenation and tandem hydroformylation–hydrogenation. Angew. Chem. Int. Ed. 48, 8022–8026 (2009).
Brown, H. C. & Zweifel, G. A stereospecific cis hydration of the double bond in cyclic derivatives. J. Am. Chem. Soc. 81, 247–247 (1959).
Bellussi, G. et al. (eds) U llmann’s Encyclopedia of Industrial Chemistry (Wiley-VCH, Hoboken, 2000).
Denard, C. A. et al. Development of a one-pot tandem reaction combining ruthenium-catalyzed alkene metathesis and enantioselective enzymatic oxidation to produce aryl epoxides. ACS Catal. 5, 3817–3822 (2015).
Zhang, W., Loebach, J. L., Wilson, S. R. & Jacobsen, E. N. Enantioselective epoxidation of unfunctionalized olefins catalyzed by salen manganese complexes. J. Am. Chem. Soc. 112, 2801–2803 (1990).
Katsuki, T. & Sharpless, K. B. The first practical method for asymmetric epoxidation. J. Am. Chem. Soc. 102, 5974–5976 (1980).
Aggarwal, V. K. & Winn, C. L. Catalytic, asymmetric sulfur ylide-mediated epoxidation of carbonyl compounds: scope, selectivity and applications in synthesis. Acc. Chem. Res. 37, 611–620 (2004).
Ito, M., Hirakawa, M., Osaku, A. & Ikariya, T. Highly efficient chemoselective hydrogenolysis of epoxides catalyzed by a (η5-C5(CH3)5)Ru complex bearing a 2-(diphenylphosphino)ethylamine ligand. Organometallics 22, 4190–4192 (2003).
Fujitsu, H., Shirahama, S., Matsumura, E., Takeshita, K. & Mochida, I. Catalytic hydrogenation of styrene oxide with cationic rhodium complexes. J. Org. Chem. 46, 2287–2290 (1981).
Murru, S., Nicholas, K. M. & Srivastava, R. S. Ruthenium(ii) sulfoxides-catalyzed hydrogenolysis of glycols and epoxides. J. Mol. Catal. A 363–364, 460–464 (2012).
Sajiki, H., Hattori, K. & Hirota, K. Pd/C(en)-catalyzed regioselective hydrogenolysis of terminal epoxides to secondary alcohols. Chem. Commun. 1041–1042 (1999).
Kwon, M. S., Park, I. S., Jang, J. S., Lee, J. S. & Park, J. Magnetically separable Pd catalyst for highly selective epoxide hydrogenolysis under mild conditions. Org. Lett. 9, 3417–3419 (2007).
Ley, S. V. et al. Recyclable polyurea-microencapsulated pd(0) nanoparticles: an efficient catalyst for hydrogenolysis of epoxides. Org. Lett. 5, 4665–4668 (2003).
Newman, M. S., Underwood, G. & Renoll, M. The reduction of terminal epoxides. J. Am. Chem. Soc. 71, 3362–3363 (1949).
O, W. W. N., Lough, A. J. & Morris, R. H. The hydrogenation of molecules with polar bonds catalyzed by a ruthenium(ii) complex bearing a chelating N-heterocyclic carbene with a primary amine donor. Chem. Commun. 46, 8240–8242 (2010).
Gansäuer, A., Fan, C.-A. & Piestert, F. Sustainable radical reduction through catalytic hydrogen atom transfer. J. Am. Chem. Soc. 130, 6916–6917 (2008).
Wenz, J., Wadepohl, H. & Gade, L. H. Regioselective hydrosilylation of epoxides catalysed by nickel(ii) hydrido complexes. Chem. Commun. 53, 4308–4311 (2017).
Gansäuer, A., Klatte, M., Brändle, G. M. & Friedrich, J. Catalytic hydrogen atom transfer (HAT) for sustainable and diastereoselective radical reduction. Angew. Chem. Int. Ed. 51, 8891–8894 (2012).
Bullock, R. M. Abundant metals give precious hydrogenation performance. Science 342, 1054–1055 (2013).
Chirik, P. & Morris, R. Getting down to earth: the renaissance of catalysis with abundant metals. Acc. Chem. Res. 48, 2495 (2015).
Chirik, P. J. Iron- and cobalt-catalyzed alkene hydrogenation: catalysis with both redox-active and strong field ligands. Acc. Chem. Res. 48, 1687–1695 (2015).
Filonenko, G. A., van Putten, R., Hensen, E. J. M. & Pidko, E. A. Catalytic (de)hydrogenation promoted by non-precious metals—Co, Fe and Mn: recent advances in an emerging field. Chem. Soc. Rev. 47, 1459–1483 (2018).
Kallmeier, F. & Kempe, R. Manganese complexes for (de)hydrogenation catalysis: a comparison to cobalt and iron catalysts. Angew. Chem. Int. Ed. 57, 46–60 (2018).
Morris, R. H. Asymmetric hydrogenation, transfer hydrogenation and hydrosilylation of ketones catalyzed by iron complexes. Chem. Soc. Rev. 38, 2282–2291 (2009).
Zell, T. & Milstein, D. Hydrogenation and dehydrogenation iron pincer catalysts capable of metal–ligand cooperation by aromatization/dearomatization. Acc. Chem. Res. 48, 1979–1994 (2015).
Chakraborty, S., Bhattacharya, P., Dai, H. & Guan, H. Nickel and iron pincer complexes as catalysts for the reduction of carbonyl compounds. Acc. Chem. Res. 48, 1995–2003 (2015).
Misal Castro, L. C., Li, H., Sortais, J.-B. & Darcel, C. When iron met phosphines: a happy marriage for reduction catalysis. Green Chem. 17, 2283–2303 (2015).
Ziebart, C. et al. Well-defined iron catalyst for improved hydrogenation of carbon dioxide and bicarbonate. J. Am. Chem. Soc. 134, 20701–20704 (2012).
Wienhöfer, G., Westerhaus, F. A., Junge, K., Ludwig, R. & Beller, M. A molecularly defined iron-catalyst for the selective hydrogenation of α,β-unsaturated aldehydes. Chem. Eur. J. 19, 7701–7707 (2013).
Rezayee, N. M., Samblanet, D. C. & Sanford, M. S. Iron-catalyzed hydrogenation of amides to alcohols and amines. ACS Catal. 6, 6377–6383 (2016).
Lagaditis, P. O. et al. Iron(ii) complexes containing unsymmetrical P–N–P′ pincer ligands for the catalytic asymmetric hydrogenation of ketones and imines. J. Am. Chem. Soc. 136, 1367–1380 (2014).
Gorgas, N., Stöger, B., Veiros, L. F. & Kirchner, K. Highly efficient and selective hydrogenation of aldehydes: a well-defined Fe(ii) catalyst exhibits noble-metal activity. ACS Catal. 6, 2664–2672 (2016).
Casey, C. P. & Guan, H. An efficient and chemoselective iron catalyst for the hydrogenation of ketones. J. Am. Chem. Soc. 129, 5816–5817 (2007).
Chakraborty, S., Brennessel, W. W. & Jones, W. D. A molecular iron catalyst for the acceptorless dehydrogenation and hydrogenation of N-heterocycles. J. Am. Chem. Soc. 136, 8564–8567 (2014).
Yu, R. P. et al. High-activity iron catalysts for the hydrogenation of hindered, unfunctionalized alkenes. ACS Catal. 2, 1760–1764 (2012).
Vyas, D. J., Larionov, E., Besnard, C., Guénée, L. & Mazet, C. Isomerization of terminal epoxides by a [Pd–H] catalyst: a combined experimental and theoretical mechanistic study. J. Am. Chem. Soc. 135, 6177–6183 (2013).
Lamb, J. R., Mulzer, M., LaPointe, A. M. & Coates, G. W. Regioselective isomerization of 2,3-disubstituted epoxides to ketones: an alternative to the wacker oxidation of internal alkenes. J. Am. Chem. Soc. 137, 15049–15054 (2015).
Jiang, G. et al. Ruthenium porphyrin-catalyzed aerobic oxidation of terminal aryl alkenes to aldehydes by a tandem epoxidation–isomerization pathway. Angew. Chem. Int. Ed. 47, 6638–6642 (2008).
Hrdina, R. et al. Silicon–(thio)urea Lewis acid catalysis. J. Am. Chem. Soc. 133, 7624–7627 (2011).
Acknowledgements
The authors thank the analytic department (LIKAT) for their support, and the State of Mecklenburg-Western Pomerania, the Federal State of Germany (BMBF) and the EU (grant 670986) for financial support. The authors also thank W. Baumann (LIKAT) for helpful discussions regarding NMR analyses and D. Leonard (LIKAT) for assistance with manuscript preparation.
Author information
Authors and Affiliations
Contributions
M.B. and W. Liu. conceived and designed the experiments. W. Liu and W. Li performed the experiments and analysed the data. A.S. performed X-ray crystal structure analyses. K.J. participated in the discussions and supported the project. M.B. and W. Liu co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, Supplementary Tables 1-6, Supplementary references
compound S7
Crystallographic Data for compound S7
compound S9
Crystallographic Data for compound S9
compound S10
Crystallographic Data for compound S10
compound 9a
Crystallographic Data for compound 9a
compound 10’
Crystallographic Data for compound 10’
Rights and permissions
About this article
Cite this article
Liu, W., Li, W., Spannenberg, A. et al. Iron-catalysed regioselective hydrogenation of terminal epoxides to alcohols under mild conditions. Nat Catal 2, 523–528 (2019). https://doi.org/10.1038/s41929-019-0286-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-019-0286-7
This article is cited by
-
Anti-Markovnikov hydrochlorination and hydronitrooxylation of α-olefins via visible-light photocatalysis
Nature Catalysis (2023)
-
Synthesis of amorphous Pd-based nanocatalysts for efficient alcoholysis of styrene oxide and electrochemical hydrogen evolution
Nano Research (2023)
-
Tunable hydrogen coverage on electron-deficient platinum nanoparticles for efficient hydrogenation reactions
Nano Research (2023)