Abstract
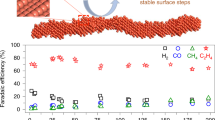
Upgrading carbon dioxide to high-value multicarbon (C2+) products is one promising avenue for fuel and chemical production. Among all the monometallic catalysts, copper has attracted much attention because of its unique ability to convert CO2 or CO into C2+ products with an appreciable selectivity. Although numerous attempts have been made to synthesize Cu materials that expose the desired facets, it still remains a challenge to obtain high-quality nanostructured Cu catalysts for the electroreduction of CO2/CO. Here we report a facile synthesis of freestanding triangular-shaped two-dimensional Cu nanosheets that selectively expose the (111) surface. In a 2 M KOH electrolyte, the Cu nanosheets exhibit an acetate Faradaic efficiency of 48% with an acetate partial current density up to 131 mA cm−2 in electrochemical CO reduction. Further analysis suggest that the high acetate selectivity is attributed to the suppression of ethylene and ethanol formation, probably due to the reduction of exposed (100) and (110) surfaces.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data sets generated during and/or analysed during the current study are available from the corresponding authors on reasonable request.
References
Zhuang, T.-T. et al. Steering post-C–C coupling selectivity enables high efficiency electroreduction of carbon dioxide to multi-carbon alcohols. Nat. Catal. 1, 421–428 (2018).
Ripatti, D. S., Veltman, T. R. & Kanan, M. W. Carbon monoxide gas diffusion electrolysis that produces concentrated C2 products with high single-pass conversion. Joule 3, 240–256 (2018).
Jouny, M., Luc, W. & Jiao, F. High-rate electroreduction of carbon monoxide to multi-carbon products. Nat. Catal. 1, 748–755 (2018).
Jiang, K. et al. Metal ion cycling of Cu foil for selective C–C coupling in electrochemical CO2 reduction. Nat. Catal. 1, 111–119 (2018).
Dinh, C.-T. et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 360, 783–787 (2018).
De Luna, P. et al. Catalyst electro-redeposition controls morphology and oxidation state for selective carbon dioxide reduction. Nat. Catal. 1, 103–110 (2018).
Ma, S. et al. Electroreduction of carbon dioxide to hydrocarbons using bimetallic Cu–Pd catalysts with different mixing patterns. J. Am. Chem. Soc. 139, 47–50 (2017).
Lu, Q. et al. A selective and efficient electrocatalyst for carbon dioxide reduction. Nat. Commun. 5, 3242 (2014).
Li, C. W., Ciston, J. & Kanan, M. W. Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper. Nature 508, 504–507 (2014).
Jouny, M., Luc, W. & Jiao, F. General techno-economic analysis of CO2 electrolysis systems. Ind. Eng. Chem. Res. 57, 2165–2177 (2018).
Lv, J.-J. et al. A highly porous copper electrocatalyst for carbon dioxide reduction. Adv. Mater. 30, 1803111 (2018).
Raciti, D., Livi, K. J. & Wang, C. Highly dense Cu nanowires for low-overpotential CO2 reduction. Nano Lett. 15, 6829–6835 (2015).
Hori, Y., Wakebe, H., Tsukamoto, T. & Koga, O. Electrocatalytic process of Co selectivity in electrochemical reduction of CO2 at metal-electrodes in aqueous-media. Electrochim. Acta 39, 1833–1839 (1994).
Kuhl, K. P., Cave, E. R., Abram, D. N. & Jaramillo, T. F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 5, 7050–7059 (2012).
Kuhl, K. P. et al. Electrocatalytic conversion of carbon dioxide to methane and methanol on transition metal surfaces. J. Am. Chem. Soc. 136, 14107–14113 (2014).
Hori, Y., Takahashi, R., Yoshinami, Y. & Murata, A. Electrochemical reduction of CO at a copper electrode. J. Phys. Chem. B 101, 7075–7081 (1997).
Yang, H.-J., He, S.-Y., Chen, H.-L. & Tuan, H.-Y. Monodisperse copper nanocubes: synthesis, self-assembly, and large-area dense-packed films. Chem. Mater. 26, 1785–1793 (2014).
Jin, M. et al. Shape-controlled synthesis of copper nanocrystals in an aqueous solution with glucose as a reducing agent and hexadecylamine as a capping agent. Angew. Chem. Int. Ed. 50, 10560–10564 (2011).
Huang, J. F. et al. Potential-induced nanoclustering of metallic catalysts during electrochemical CO2 reduction. Nat. Commun. 9, 3117 (2018).
Guo, H. Z. et al. Shape-selective formation of monodisperse copper nanospheres and nanocubes via disproportionation reaction route and their optical properties. J. Phys. Chem. C 118, 9801–9808 (2014).
Salzemann, C., Urban, J., Lisiecki, I. & Pileni, M. P. Characterization and growth process of copper nanodisks. Adv. Funct. Mater. 15, 1277–1284 (2005).
Tao, F. et al. Break-up of stepped platinum catalyst surfaces by high CO coverage. Science 327, 850–853 (2010).
Tao, F. et al. Reaction-driven restructuring of Rh–Pd and Pt–Pd core–shell nanoparticles. Science 322, 932–934 (2008).
Verma, S. et al. Insights into the low overpotential electroreduction of CO2 to CO on a supported gold catalyst in an alkaline flow electrolyzer. ACS Energy Lett. 3, 193–198 (2018).
Zhuang, T.-T. et al. Copper nanocavities confine intermediates for efficient electrosynthesis of C3 alcohol fuels from carbon monoxide. Nat. Catal. 1, 946–951 (2018).
Ma, S. C. et al. One-step electrosynthesis of ethylene and ethanol from CO2 in an alkaline electrolyzer. J. Power Sources 301, 219–228 (2016).
Weekes, D. M., Salvatore, D. A., Reyes, A., Huang, A. X. & Berlinguette, C. P. Electrolytic CO2 reduction in a flow cell. Acc. Chem. Res. 51, 910–918 (2018).
Dunwell, M., Luc, W., Yan, Y. S., Jiao, F. & Xu, B. J. Understanding surface-mediated electrochemical reactions: CO2 reduction and beyond. ACS Catal. 8, 8121–8129 (2018).
Lum, Y. W., Cheng, T., Goddard, W. A. & Ager, J. W. Electrochemical CO reduction builds solvent water into oxygenate products. J. Am. Chem. Soc. 140, 9337–9340 (2018).
Liu, X. Y. et al. pH effects on the electrochemical reduction of CO2 towards C2 products on stepped copper. Nat. Commun. 10, 32 (2019).
Wang, L. et al. Electrochemical carbon monoxide reduction on polycrystalline copper: effects of potential, pressure, and pH on selectivity toward multicarbon and oxygenated products. ACS Catal. 8, 7445–7454 (2018).
Strmcnik, D. et al. Improving the hydrogen oxidation reaction rate by promotion of hydroxyl adsorption. Nat. Chem. 5, 300–306 (2013).
Raciti, D. et al. Low-overpotential electroreduction of carbon monoxide using copper nanowires. ACS Catal. 7, 4467–4472 (2017).
Droog, J. M. M. & Schlenter, B. Oxygen electrosorption on copper single-crystal electrodes in sodium-hydroxide solution. J. Electroanal. Chem. 112, 387–390 (1980).
Schouten, K. J. P., Gallent, E. P. & Koper, M. T. M. Structure sensitivity of the electrochemical reduction of carbon monoxide on copper single crystals. ACS Catal. 3, 1292–1295 (2013).
Hahn, C. et al. Engineering Cu surfaces for the electrocatalytic conversion of CO2: controlling selectivity toward oxygenates and hydrocarbons. Proc. Natl Acad. Sci. USA 114, 5918–5923 (2017).
Cheng, T., Xiao, H. & Goddard, W. A. Full atomistic reaction mechanism with kinetics for CO reduction on Cu(100) from ab initio molecular dynamics free-energy calculations at 298 K. Proc. Natl Acad. Sci. USA 114, 1795–1800 (2017).
Cheng, T., Xiao, H. & Goddard, W. A. Nature of the active sites for CO reduction on copper nanoparticles; suggestions for optimizing performance. J. Am. Chem. Soc. 139, 11642–11645 (2017).
Xiao, H., Cheng, T., Goddard, W. A. & Sundararaman, R. Mechanistic explanation of the pH dependence and onset potentials for hydrocarbon products from electrochemical reduction of CO on Cu(111). J. Am. Chem. Soc. 138, 483–486 (2016).
Calle-Vallejo, F. & Koper, M. T. M. Theoretical considerations on the electroreduction of CO to C2 species on Cu(100) electrodes. Angew. Chem. Int. Ed. 52, 7282–7285 (2013).
Chen, C. et al. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science 343, 1339–1343 (2014).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Scott, A. P. & Radom, L. Harmonic vibrational frequencies: an evaluation of Hartree−Fock, Møller−Plesset, quadratic configuration interaction, density functional theory, and semiempirical scale factors. J. Phys. Chem. 100, 16502–16513 (1996).
Peterson, A. A., Abild-Pedersen, F., Studt, F., Rossmeisl, J. & Nørskov, J. K. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci. 3, 1311–1315 (2010).
Henkelman, G., Uberuaga, B. P. & Jonsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Acknowledgements
The work is supported by the Department of Energy (USA) under Award no. DE-FE0029868 and the National Natural Science Foundation of China under Award nos 51601030 and 21773023. F.J., W.L., J.-J.L., M.J. and B.H.K. also thank the National Science Foundation Faculty Early Career Development program (Award no. CBET-1350911). Y.K. and X.F. acknowledge the support from International Institute for Nanotechnology (IIN) and Institute for Sustainability and Energy (ISEN) at Northwestern University. The theoretical calculation is supported by the Welch Foundation (Grant no. F-1959-20180324) and the startup grant from UT Austin, and used computational resources sponsored by the DOE’s Office of Energy Efficiency and Renewable Energy and located at the National Renewable Energy Laboratory, and the Texas Advanced Computing Center (TACC) at UT Austin. This work made use of the Electron Probe Instrumentation Center (EPIC) facility of Northwestern University’s Atomic and Nanoscale Characterization Experimental Center (NUANCE), which has received support from the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCS-1542205); the Materials Research Science and Engineering Centers (MRSEC) program (NSF DMR-1121262) at the Materials Research Center; the IIN. This work made use of the J.B. Cohen X-Ray Diffraction Facility supported by MRSEC and SHyNE. The authors acknowledge D. Su (Brookhaven National Laboratory), X. Ye (Indiana University) and A. Petford-Long (Northwestern University) for help in the discussion. This research used resources at the 8-ID Beamline of the National Synchrotron Light Source II, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract no. DE-SC0012704. The authors acknowledge E. Stavitski (8-ID Beamline, NSLS-II, Brookhaven National Laboratory) for assistance in the XAS measurements.
Author information
Authors and Affiliations
Contributions
Y.K. and F.J. conceived the idea and supervised the project. X.F. designed the catalyst and synthesized the Cu nanomaterials. W.L. performed the electrocatalytic studies. Y.K., X.F., W.L., Y.L. and F.J. analysed the data and drafted the manuscript. J.-J.L. and M.J, performed the electrocatalytic study on the Cu nanoparticles and micrometre-sized particles. M.J. designed the operando XAS flow-cell electrolyser, and M.J., B.H.K. and W.L. performed the XAS study. Y.X., X.H. and J.W. facilitated the electron microscopic work. Q.T. assisted the AFM measurement. J.S. and Y.L. performed the computational modelling studies. All the authors contributed to the discussion of the results and manuscript preparation. W.L. and X.F. contributed equally to this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–24 and Supplementary Table 1.
Supplementary Data 1
Atomic coordinates of the optimized computational models.
Rights and permissions
About this article
Cite this article
Luc, W., Fu, X., Shi, J. et al. Two-dimensional copper nanosheets for electrochemical reduction of carbon monoxide to acetate. Nat Catal 2, 423–430 (2019). https://doi.org/10.1038/s41929-019-0269-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-019-0269-8
This article is cited by
-
Site-selective protonation enables efficient carbon monoxide electroreduction to acetate
Nature Communications (2024)
-
Unraveling the rate-determining step of C2+ products during electrochemical CO reduction
Nature Communications (2024)
-
A silver–copper oxide catalyst for acetate electrosynthesis from carbon monoxide
Nature Synthesis (2023)
-
Oxidation of metallic Cu by supercritical CO2 and control synthesis of amorphous nano-metal catalysts for CO2 electroreduction
Nature Communications (2023)
-
Accelerating electrochemical CO2 reduction to multi-carbon products via asymmetric intermediate binding at confined nanointerfaces
Nature Communications (2023)