Abstract
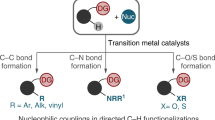
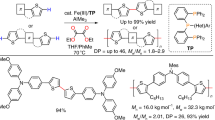
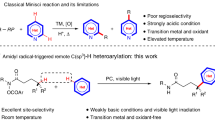
Twofold C–H activation/cross-coupling of stoichiometric amounts of organic molecules, R1–H and R2–H, to form an R1–R2 product free of homocoupling products is a goal in the activation of unreactive C–H bonds, as it will dramatically simplify organic synthesis. No reliable strategy to eliminate the homocoupling side products effectively without recourse to the use of an excess of one reactant over another is known. We report herein that a transient connection of two reactants by an anionic group appended to one reactant achieves this goal under mildly oxidative iron-catalysed conditions, through the formation of a productive heteroleptic R1–M–R2 intermediate. We utilized an N-(quinolin-8-yl)amide anion for the temporary connection and cross-coupled a stoichiometric mixture of aromatics in high yield without any trace of homocoupling products. A short-step synthesis of several donor/acceptor thiophene compounds and carbon/sulfur-bridged flat conjugated systems illustrates the utility of this method to streamline organic synthesis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the findings of this study, including experimental procedures and compound characterization, are available within the paper and its Supplementary Information, or from the authors upon reasonable request.
References
Murai, S. et al. Efficient catalytic addition of carbon–hydrogen bonds to olefins. Nature 366, 529–531 (1993).
Arndtsen, B. A., Bergman, R. G., Mobley, T. A. & Peterson, T. H. Selective intermolecular carbon–hydrogen bond activation by synthetic metal complexes in homogeneous solution. Acc. Chem. Res. 28, 154–162 (1995).
Shilov, A. E. & Shul’pin, G. B. Activation of C–H bonds by metal complexes. Chem. Rev. 97, 2879–2932 (1997).
Crabtree, R. H. & Lei, A. Introduction: CH activation. Chem. Rev. 117, 8481–8482 (2017).
Dong, Z., Ren, Z., Thompson, S. J., Xu, Y. & Dong, G. Transition-metal catalyzed alkylation using alkenes. Chem. Rev. 117, 9333–9403 (2017).
Lyons, T. W. & Sanford, M. S. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem. Rev. 110, 1147–1169 (2010).
Roudesly, F., Oble, J. & Poli, G. Metal-catalyzed C–H activation/functionalization: the fundamentals. J. Mol. Catal. A 426, 275–296 (2017).
Kuhl, N., Hopkinson, M. N., Wencel-Delord, J. & Glorius, F. Beyond directing groups: transition-metal-catalyzed C–H activation of simple arenes. Angew. Chem. Int. Ed. 51, 10236–10254 (2012).
Chen, X., Engle, K. M., Wang, D.-H. & Yu, J.-Q. Palladium(ii)-catalyzed C–H activation/C–C cross-coupling reactions: versatility and practicality. Angew. Chem. Int. Ed. 48, 5094–5115 (2009).
Yang, Y., Lan, J. & You, J. Oxidative C–H/C–H coupling reactions between two (hetero)arenes. Chem. Rev. 117, 8787–8863 (2017).
Cho, S. H., Kim, J. Y., Kwak, J. & Chang, S. Recent advances in the transition metal-catalyzed twofold oxidative C–H bond activation strategy for C–C and C–N bond formation. Chem. Soc. Rev. 40, 5068–5083 (2011).
Stuart, D. R. & Fagnou, K. The catalytic cross-coupling of unactivated arenes. Science 316, 1172–1175 (2007).
Xi, P. et al. Palladium(ii)-catalyzed oxidative C–H/C–H cross-coupling of heteroarenes. J. Am. Chem. Soc. 132, 1822–1824 (2010).
Brasche, G., García-Fortanet, J. & Buchwald, S. L. Twofold C–H functionalization: palladium-catalyzed ortho arylation of anilides. Org. Lett. 10, 2207–2210 (2008).
Hull, K. L. & Sanford, M. S. Catalytic and highly regioselective cross-coupling of aromatic C–H substrates. J. Am. Chem. Soc. 129, 11904–11905 (2007).
Wang, X., Leow, D. & Yu, J.-Q. Pd(ii)-catalyzed para-selective C–H arylation of monosubstituted arenes. J. Am. Chem. Soc. 133, 13864–13867 (2011).
Aihira, Y., Tobisu, M., Fukumoto, Y. & Chatani, N. Ni(ii)-catalyzed oxidative coupling between C(sp 2)–H in benzamides and C(sp 3)–H in toluene derivatives. J. Am. Chem. Soc. 136, 15509–15512 (2014).
Cambeiro, X. C., Ahlsten, N. & Larrosa, I. Au-catalyzed cross-coupling of arenes via double C–H activation. J. Am. Chem. Soc. 137, 15636–15639 (2015).
Zhang, L. et al. Experimental and theoretical studies on Ru(ii)-catalyzed oxidative C−H/C−H coupling of phenols with aromatic amides using air as oxidant: scope, synthetic applications, and mechanistic insights. ACS Catal. 8, 8324–8335 (2018).
Masui, K., Ikegami, H. & Mori, A. Palladium-catalyzed C–H homocoupling of thiophenes: facile construction of bithiophene structure. J. Am. Chem. Soc. 126, 5074–5075 (2004).
Shang, R., Ilies, L. & Nakamura, E. Iron-catalyzed C–H bond activation. Chem. Rev. 117, 9086–9139 (2017).
Cera, G. & Ackermann, L. Iron-catalyzed C–H functionalization processes. Top. Curr. Chem. 374, 191–224 (2016).
Shang, R., Ilies, L., Asako, S. & Nakamura, E. Iron-catalyzed C(sp 2)–H bond functionalization with organoboron compounds. J. Am. Chem. Soc. 136, 14349–14352 (2014).
Shang, R., Ilies, L. & Nakamura, E. Iron-catalyzed directed C(sp 2)–H and C(sp 3)–H functionalization with trimethylaluminum. J. Am. Chem. Soc. 137, 7660–7663 (2015).
Shang, R., Ilies, L. & Nakamura, E. Iron-catalyzed ortho C–H methylation of aromatics bearing a simple carbonyl group with methylaluminum and tridentate phosphine ligand. J. Am. Chem. Soc. 138, 10132–10135 (2016).
Daugulis, O., Roane, J. & Tran, D. Bidentate, monoamionic auxiliary-directed functionalization of carbon–hydrogen bonds. Acc. Chem. Res. 48, 1053–1064 (2015).
Shang, R., Ilies, L., Matsumoto, A. & Nakamura, E. β-Arylation of carboxamides via iron-catalyzed C(sp 3)–H bond activation. J. Am. Chem. Soc. 135, 6030–6032 (2013).
Asako, S., Ilies, L. & Nakamura, E. Iron-catalyzed ortho-allylation of aromatic carboxamides with allyl ethers. J. Am. Chem. Soc. 135, 17755–17757 (2013).
Tang, D.-T. D., Collins, K. D., Ernst, J. B. & Glorius, F. Pd/C as a catalyst for completely regioselective C–H functionalization of thiophenes under mild conditions. Angew. Chem. Int. Ed. 53, 1809–1813 (2014).
Colletto, C., Panigrahi, A., Fernández-Casado, J. & Larrosa, I. Ag(i)–C–H activation enables near-room-temperature direct α-arylation of benzo[b]thiophenes. J. Am. Chem. Soc. 140, 9638–9643 (2018).
Wong, K.-T. et al. Synthesis and structure of novel heteroarene-fused coplanar, π-conjugated chromophores. Org. Lett. 8, 5033–5036 (2006).
Zhu, X., Tsuji, H., Navarrete, J. T. L., Casado, J. & Nakamura, E. Carbon-bridged oligo(phenylenevinylene)s: stable p-systems with high responsiveness to doping and excitation. J. Am. Chem. Soc. 134, 19254–19259 (2012).
Molina-Ontoria, A. et al. Benzotrithiophene-based hole-transporting materials for 18.2% perovskite solar cells. Angew. Chem. Int. Ed. 55, 6270–6274 (2016).
Nagano, T. & Hayashi, T. Iron-catalyzed oxidative homo-coupling of aryl Grignard reagents. Org. Lett. 7, 491–493 (2005).
Yanagisawa, S., Ueda, K., Sekizawa, H. & Itami, K. Programmed synthesis of tetraarylthiophenes through sequential C−H arylation. J. Am. Chem. Soc. 131, 14622–14623 (2009).
Ilies, L., Ichikawa, S., Asako, S., Matsubara, T. & Nakamura, E. Iron-catalyzed directed alkylation of alkenes and arenes with alkylzinc halides. Adv. Synth. Catal. 357, 2175–2179 (2015).
Shen, K., Fu, Y., Li, J.-N., Liu, L. & Guo, Q.-X. What are the pK a values of C–H bonds in aromatic heterocyclic compounds in DMSO? Tetrahedron 63, 1568–1576 (2007).
Sawamura, Y. et al. Iron-catalyzed Friedel–Crafts benzylation with benzyl TMS ethers at room temperature. Chem. Eur. J. 20, 510–516 (2014).
Ibanez, J. G. et al. Conducting polymers in the fields of energy, environmental remediation, and chemical–chiral sensors. Chem. Rev. 118, 4731–4816 (2018).
Guo, Y. et al. Citric acid modulated growth of oriented lead perovskite crystals for efficient solar cells. J. Am. Chem. Soc. 139, 9598–9604 (2017).
Groenendaal, L., Jonas, F., Freitag, D., Pielartzik, H. & Reynolds, J. R. Poly(3,4-ethylenedioxythiophene) and its derivatives: past, present, and future. Adv. Mater. 12, 481–494 (2000).
Osaka, I. et al. Synthesis, characterization, and transistor and solar cell applications of a naphthobisthiadiazole-based semiconducting polymer. J. Am. Chem. Soc. 134, 3498–3507 (2012).
Sun, Y. et al. Two-state reactivity in low-valent iron-mediated C–H activation and the implications for other first-row transition metals. J. Am. Chem. Soc. 138, 3715–3730 (2016).
Acknowledgements
The authors thank MEXT for financial support (KAKENHI Grant-in-Aid for Scientific Research (S) 15H05754 to E.N., JP18H04238 in Precisely Designed Catalysts with Customized Scaffolding to L.I. and KAKENHI Grant-in-Aid for Young Scientists (B) JP17K14480 to R.S.).
Author information
Authors and Affiliations
Contributions
E.N. and R.S. guided the research and wrote the manuscript. T.D. performed the experiments to study the scope, application and mechanism. All authors contributed to designing the experiments, analysing the data and editing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information
Supplementary Methods, Supplementary Figures 1–4, Supplementary References
Rights and permissions
About this article
Cite this article
Doba, T., Matsubara, T., Ilies, L. et al. Homocoupling-free iron-catalysed twofold C–H activation/cross-couplings of aromatics via transient connection of reactants. Nat Catal 2, 400–406 (2019). https://doi.org/10.1038/s41929-019-0245-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-019-0245-3
This article is cited by
-
Iron-catalysed regioselective thienyl C–H/C–H coupling
Nature Catalysis (2021)