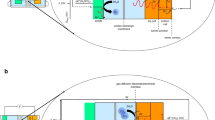
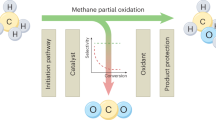
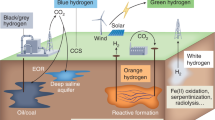
Abstract
The Sabatier reaction (that is, CO2 methanation) is undergoing a revival for two main reasons. First, the power-to-gas concept offers the prospect of large-scale recycling of (point source) CO2 emissions, in combination with the use of large quantities of renewable energy to form methane. When this can be achieved in a cost-effective manner, it can use the gas distribution infrastructure that already exists. However, methanation is no simple panacea to the detrimental environmental effect of CO2 emissions, and reaction products other than methane should also be targeted. Second, methanation has been identified as an important reaction to facilitate long-term space exploration missions by space agencies, such as NASA. This Perspective discusses the current understanding of CO2 hydrogenation within these concepts, from fundamental mechanistic aspects to several parameters that will ultimately define its technical and economic feasibility on Earth and in space, as we transition into the era of small-molecule activation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Senderens, J.-B. & Sabatier, P. Nouvelles synthèses du méthane. Comptes Rendus Acad. Sci. 82 514–516 (1902). .
Sabatier, P. & Reid, E. E. Catalysis in Organic Chemistry. 2nd edn, (D. van Nostrand Company, New York, 1923).
Sabatier, P. & Senderens, J.-B. Hydrogénation directe des oxydes du carbone en présence de divers métaux divisés. Comptes Rendus Acad. Sci. 134, 689–691 (1903).
Artz, J. et al. Sustainable conversion of carbon dioxide: an integrated review of catalysis and life cycle assessment. Chem. Rev. 118, 434–504 (2018).
Rönsch, S. et al. Review on methanation — from fundamentals to current projects. Fuel 166, 276–296 (2016).
Álvarez, A. et al. CO2 activation over catalytic surfaces. ChemPhysChem 18, 3135–3141 (2017).
Prieto, G. Carbon dioxide hydrogenation into higher hydrocarbons and oxygenates: thermodynamic and kinetic bounds and progress with heterogeneous and homogeneous catalysis. ChemSusChem 10, 1056–1070 (2017).
Yang, H. et al. A review of catalytic hydrogenation of carbon dioxide into value-added hydrocarbons. Catal. Sci. Technol. 7, 4580–4598 (2017).
Kattel, S., Liu, P. & Chen, J. G. Tuning selectivity of CO2 hydrogenation reactions at the metal/oxide interface. J. Am. Chem. Soc. 139 9739–9754 (2017).
Rogelj, J. et al. Differences between carbon budget estimates unravelled. Nat. Clim. Change 6, 245–252 (2016).
Hausfather, F. Analysis: how much ‘carbon budget’ is left to limit global warming to 1.5C? Carbon Brief (9 April 2018); https://www.carbonbrief.org/analysis-how-much-carbon-budget-is-left-to-limit-global-warming-to-1-5c
Ricke, K. L. & Caldeira, K. Maximum warming occurs about one decade after a carbon dioxide emission. Environ. Res. Lett. 9, 124002–124010 (2014).
Schlögl, R. The revolution continues: energiewende 2.0. Angew. Chem. Int. Ed. 54, 4436–4439 (2015).
van Vuuren, D. P. et al. Alternative pathways to the 1.5 °C target reduce the need for negative emission technologies. Nat. Clim. Change 8, 391–397 (2018).
Shell Scenarios: Sky — Meeting the Goals of the Paris Agreement (Shell, 2018).
Anderson, K. & Peters, G. The promise of negative emissions. Science 354, 354–355 (2010).
Kramer, G. J. & Haigh, M. No quick switch to low-carbon energy. Nature 462, 568–569 (2009).
Majumdar, A. & Deutch, J. M. Research opportunities for CO2 utilization and negative emissions at the gigatonne-scale. Joule 2, 805–809 (2018).
Bui, M. et al. Carbon capture and storage (CCS): the way forward. Energy Environ. Sci. 11, 1062–1176 (2018).
IPCC Carbon Dioxide Capture and Storage (eds Metz, B., Davidson, O., de Coninck, H. C., Loos, M. & Meyer, L. A.) (Cambridge Univ. Press, 1975).
Kim, S. M. et al. Integrated CO2 capture and conversion as an efficient process for fuels from greenhouse gases. ACS Catal. 8, 2815–2823 (2018).
Stolaroff, J. K., Lowry, G. V. & Keith, D. W. Using CaO- and MgO-rich industrial waste streams for carbon sequestration. Energy Convers. Manag. 46, 687–699 (2005).
House, K. Z. et al. Economic and energetic analysis of capturing CO2 from ambient air. Proc. Natl Acad. Sci. USA 108, 20428–20433 (2011).
Koytsoumpa, E. I., Bergins, C. & Kakaras, E. The CO2 economy: review of CO2 capture and reuse technologies. J. Supercrit. Fluids 132, 3–16 (2018).
Keith, D. W., Holmes, G., St. Angelo, D. & Heidel, K. A Process for capturing CO2 from the atmosphere. Joule 2, 1573–1594 (2018).
Schlögl, R. E. Mobility and the energy transition. Angew. Chem. Int. Ed. 56, 11019–11022 (2017).
Heide, D. et al. Seasonal optimal mix of wind and solar power in a future, highly renewable Europe. Renew. Energy 35, 2483–2489 (2010).
Götz, M. et al. Renewable power-to-gas: a technological and economic review. Renew. Energy 85, 1371–1390 (2016).
Gonzalez-Salazar, M. A., Kirsten, T. & Prchlik, L. Review of the operational flexibility and emissions of gas- and coal-fired power plants in a future with growing renewables. Renew. Sustain. Energy Rev. 82, 1497–1513 (2018).
Benchmarking of large scale hydrogen underground storage with competing options (HyUnder, 2014).
Zhang, C. Hydrogen storage: improving reversibility. Nat. Energy 2, 17064 (2017).
Aakko-Saksa, P. T., Cook, C., Kiviaho, J. & Repo, T. Liquid organic hydrogen carriers for transportation and storing of renewable energy — review and discussion. J. Power Sources 396, 803–823 (2018).
Younas, M. et al. Recent advancements, fundamental challenges, and opportunities in catalytic methanation of CO2. Energy Fuels 30, 8815–8831 (2016).
Audi e-gas plant qualified to participate in balancing market to stabilize grid. Green Car Congress (15 July 2015); https://www.greencarcongress.com/2015/07/20150715-egas.html
The Sabatier system. NASA (12 May 2011); https://www.nasa.gov/mission_pages/station/research/news/sabatier.html
Muscatello, A. & Santiago-Maldonado, E. Mars in situ resource utilization technology evaluation. In American 50th AIAA Aeorospace Sciences Meeting https://doi.org/10.2514/6.2012-360 (AIAA, 2012).
Wang, W., Wang, S., Ma, X. & Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 40, 3703–3727 (2011).
Munnik, P., Velthoen, M. E. Z., de Jongh, P. E., De Jong, K. P. & Gommes, C. J. Nanoparticle growth in supported nickel catalysts during methanation reaction-larger is better. Angew. Chem. Int. Ed. 53, 9493–9497 (2014).
Vogt, C. et al. Unravelling structure sensitivity in CO2 hydrogenation over nickel. Nat. Catal. 1, 127–134 (2018).
Heine, C., Lechner, B. A. J., Bluhm, H. & Salmeron, M. Recycling of CO2: probing the chemical state of the Ni(111) surface during the methanation reaction with ambient-pressure X-ray photoelectron spectroscopy. J. Am. Chem. Soc. 138, 13246–13252 (2016).
Silaghi, M., Comas-Vives, A. & Copéret, C. CO2 activation on Ni/γ−Al2O3 catalysts by first-principles calculations: from ideal surfaces to supported nanoparticles. ACS Catal. 6, 4501–4505 (2016).
Weatherbee, G. D. & Bartholomew, C. H. Hydrogenation of CO2 on group VIII metals II. Kinetics and mechanism on nickel. J. Catal. 77, 460–472 (1982).
Avanesian, T. et al. Quantitative and atomic-scale view of CO-induced Pt nanoparticle surface reconstruction at saturation coverage via DFT calculations coupled with in situ TEM and IR. J. Am. Chem. Soc. 139, 4551–4558 (2017).
Studt, F. et al. Discovery of a Ni-Ga catalyst for carbon dioxide reduction to methanol. Nat. Chem. 6, 320–324 (2014).
Wang, W. H., Himeda, Y., Muckerman, J. T., Manbeck, G. F. & Fujita, E. CO2 hydrogenation to formate and methanol as an alternative to photo- and electrochemical CO2 reduction. Chem. Rev. 115, 12936–12973 (2015).
Kunkes, E. L., Studt, F., Abild-Pedersen, F., Schlögl, R. & Behrens, M. Hydrogenation of CO2 to methanol and CO on Cu/ZnO/Al2O3: is there a common intermediate or not? J. Catal. 328, 43–48 (2015).
Kitamura Bando, K., Sayama, K., Kusuma, H., Okabe, K. & Arakawa, H. In-situ FT-IR study on CO2 hydrogenation over Cu catalysts supported on SiO2, Al2O3, and TiO2. Appl. Catal. A 165, 391–409 (1997).
Andersson, M. P. et al. Structure sensitivity of the methanation reaction: H2-induced CO dissociation on nickel surfaces. J. Catal. 255, 6–19 (2008).
Abdel-Mageed, A. M., Eckle, S., Anfang, H. G. & Behm, R. J. Selective CO methanation in CO2-rich H2 atmospheres over a Ru/zeolite catalyst: the influence of catalyst calcination. J. Catal. 298, 148–160 (2013).
Galletti, C., Specchia, S., Saracco, G. & Specchia, V. CO-selective methanation over Ru-γ-Al2O3 catalysts in H2-rich gas for PEM FC applications. Chem. Eng. Sci. 65, 590–596 (2010).
Iablokov, V. et al. Size-controlled model Co nanoparticle catalysts for CO2 hydrogenation: synthesis, characterization, and catalytic reactions. Nano Lett. 12, 3091–3096 (2012).
Sehested, J., Dahl, S., Jacobsen, J. & Rostrup-Nielsen, J. R. Methanation of CO over nickel: mechanism and kinetics at high H2/CO ratios. J. Phys. Chem. B 109, 2432–2438 (2005).
Zhu, W. et al. Active and selective conversion of CO2 to CO on ultrathin Au nanowires. J. Am. Chem. Soc. 136, 16132–16135 (2014).
Crampton, A. S. et al. Structure sensitivity in the nonscalable regime explored via catalysed ethylene hydrogenation on supported platinum nanoclusters. Nat. Commun. 7, 10389 (2016).
Yang, H. B. et al. Atomically dispersed Ni(i) as the active site for electrochemical CO2 reduction. Nat. Energy 3, 140–147 (2018).
Van Helden, P., Ciobica, I. M. & Coetzer, R. L. J. The size-dependent site composition of FCC cobalt nanocrystals. Catal. Today 261, 48–59 (2016).
Cargnello, M. et al. Control of metal nanocrystal size reveals metal-support interface role for ceria catalysts. Science 341, 771–773 (2013).
Larmier, K. et al. CO2-to-methanol hydrogenation on zirconia-supported copper nanoparticles: reaction intermediates and the role of the metal–support interface. Angew. Chem. Int. Ed. 56, 2318–2323 (2017).
Kattel, S. et al. CO2 hydrogenation over oxide-supported PtCo catalysts: the role of the oxide support in determining the product selectivity. Angew. Chem. Int. Ed. 55, 7968–7973 (2016).
Li, S. et al. Tuning the selectivity of the catalytic CO2 hydrogenation reaction by strong metal–support interaction. Angew. Chem. Int. Ed. 56, 10761–10765 (2017).
Abate, S. et al. Catalytic performance of γ–Al2O3–ZrO2–TiO2–CeO2 composite oxide supported Ni-based catalysts for CO2 methanation. Ind. Eng. Chem. Res. 55, 4451–4460 (2016).
Mebrahtu, C. et al. Hydrotalcite based Ni-Fe/(Mg, Al)Ox catalysts for CO2 methanation-tailoring Fe content for improved CO dissociation, basicity, and particle size. Catal. Sci. Technol. 8, 1016–1027 (2018).
Aldana, P. A. U. et al. Catalytic CO2 valorization into CH4 on Ni-based ceria-zirconia. Reaction mechanism by operando IR spectroscopy. Catal. Today 215, 201–207 (2013).
Mutz, B. et al. Potential of an alumina-supported Ni3Fe catalyst in the methanation of CO2: impact of alloy formation on activity and stability. ACS Catal. 7, 6802–6814 (2017).
Kustov, A. L. et al. CO methanation over supported bimetallic Ni-Fe catalysts: from computational studies towards catalyst optimization. Appl. Catal. A 320, 98–104 (2007).
Sehested, J. et al. Discovery of technical methanation catalysts based on computational screening. Top. Catal. 45, 9–13 (2007).
Liang, B. et al. Promoting role of potassium in the reverse water gas shift reaction on Pt/mullite catalyst. Catal. Today 281, 319–326 (2017).
Cybulskis, V. J., Wang, J., Pazmiño, J. H., Ribeiro, F. H. & Delgass, W. N. Isotopic transient studies of sodium promotion of Pt/Al2O3 for the water-gas shift reaction. J. Catal. 339, 163–172 (2016).
Zhou, M. & Liu, B. DFT investigation on the competition of the water–gas shift reaction versus methanation on clean and potassium-modified nickel(111) surfaces. ChemCatChem 7, 3928–3935 (2015).
Ertl, G., Knözinger, H., Schüth, F. & Weitkamp, J. Handbook of Heterogeneous Catalysis 2nd edn, (Wiley-VCH, Weinheim, 2008).
Hou, C. T. Handbook of Industrial Biocatalysis (CRC Press, Boca Raton, 2005).
Hori, Y. in Modern Aspects of Electrochemistry Vol. 42 (eds Vayenas C. G., White R. E. & Gamboa-Aldeco M. E.) 89–189 (Springer, 2008).
Feng, J., Zeng, S., Feng, J., Dong, H. & Zhang, X. CO2 electroreduction in ionic liquids: a review. Chin. J. Chem. 36, 961–970 (2018).
Dinh, C.-T. et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 360, 783–787 (2018).
Xin, L. et al. Electricity storage in biofuels: selective electrocatalytic reduction of levulinic acid to valeric acid or γ–valerolactone. ChemSusChem 6, 674–686 (2013).
Qiu, Y. et al. Integrated electrocatalytic processing of levulinic acid and formic acid to produce biofuel intermediate valeric acid. Green Chem. 16, 1305–1315 (2014).
Kim, H. J., Lee, J., Green, S. K., Huber, G. W. & Kim, W. B. Selective glycerol oxidation by electrocatalytic dehydrogenation. ChemSusChem 7, 1051–1054 (2014).
Kim, H. J. et al. Efficient eletrooxidation of biomass-derived glycerol over a graphene-supported PtRu electrocatalyst. Electrochem. Commun. 13, 890–893 (2011).
Hahn, C. et al. Engineering Cu surfaces for the electrocatalytic conversion of CO2: controlling selectivity toward oxygenates and hydrocarbons. Proc. Natl Acad. Sci. USA 114, 5918–5923 (2017).
Ni, Y. et al. Selective conversion of CO2 and H2 into aromatics. Nat. Commun. 9, 3457 (2018).
Bai, S. et al. Highly active and selective hydrogenation of CO2 to ethanol by ordered Pd-Cu nanoparticles. J. Am. Chem. Soc. 139, 6827–6830 (2017).
Kowalczyk, Z. et al. Supported ruthenium catalysts for selective methanation of carbon oxides at very low COx/H2 ratios. Appl. Catal. A 342, 35–39 (2008).
Fechete, I. Paul Sabatier — the father of the chemical theory of catalysis. Comptes Rendus Chim. 19, 1374–1381 (2016).
Guidance for Transporting Ammonia by Rail (European Fertilizer Manufacturer Association, 2007).
Furler, P. et al. Solar kerosene from H2O and CO2. In AIP Conf. Proc. 1850, 100006 (2017).
Martin, N. M. et al. Structure–function relationship during CO2 methanation over Rh/Al2O3 and Rh/SiO2 catalysts under atmospheric pressure conditions. Catal. Sci. Technol. 8, 2686–2696 (2018).
Marwood, M., Doepper, R. & Renken, A. In-situ surface and gas phase analysis for kinetic studies under transient conditions — the catalytic hydrogenation of CO2. Appl. Catal. A 151, 223–246 (1997).
Acknowledgements
This work is supported by the Netherlands Organisation for Scientific Research (NWO) Gravitation program, Netherlands Center for Multiscale Catalytic Energy Conversion (MCEC), the Advanced Research Center Chemical Building Blocks Consortium (ARC CBBC) as well as from NWO in the form of a TA-CHIPP grant. H. Wiersma (Utrecht University) is acknowledged for his literature search and executing exploratory calculations.
Author information
Authors and Affiliations
Contributions
C.V. and B.M.W. conceived the theme. C.V., M.M. and B.M.W. wrote the manuscript and designed the schemes and figures. G.J.K. contributed with insights and discussions. All authors contributed data and insights, discussed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–2, Supplementary References
Rights and permissions
About this article
Cite this article
Vogt, C., Monai, M., Kramer, G.J. et al. The renaissance of the Sabatier reaction and its applications on Earth and in space. Nat Catal 2, 188–197 (2019). https://doi.org/10.1038/s41929-019-0244-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-019-0244-4