Abstract
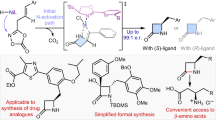
Chiral γ-lactams are effective structural motifs found in numerous pharmaceutical agents. Despite their importance, current approaches mostly necessitate laborious synthetic steps employing pre-functionalized starting materials under demanding conditions. In this regard, asymmetric C−H amidation can provide an ideal platform for rapid construction of this valuable scaffold from unactivated materials, but unsolved issues have hampered the strategy. Here, we report iridium catalysts that overcome these challenges by utilizing chiral hydrogen-bond-donor ligands. The protocol makes use of easily accessible substrates derived from carboxylic acid, which display excellent efficiency and enantioselectivity towards direct amidation of prochiral sp3 C−H bonds. Desymmetrization of meso-substrates is also achieved, where two consecutive stereogenic centres are selectively introduced in a single transformation. Computational investigations reveal the presence of crucial hydrogen bonding in the stereo-determining transition states and spectroscopic analysis of the structural analogues further corroborate this non-covalent interaction.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The X-ray crystallographic coordinates for the structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition nos. 1873622–1873624 and 1884674. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. All other data are available from the authors upon reasonable request.
References
Fisher, J. F., Meroueh, S. O. & Mobashery, S. Bacterial resistance to β-lactam antibiotics: compelling opportunism, compelling opportunity. Chem. Rev. 105, 395–424 (2005).
Caruano, J., Muccioli, G. G. & Robiette, R. Biologically active γ-lactams: synthesis and natural sources. Org. Biomol. Chem. 14, 10134–10156 (2016).
Ye, L.-W., Shu, C. & Gagosz, F. Recent progress towards transition metal-catalyzed synthesis of γ-lactams. Org. Biomol. Chem. 12, 1833–1845 (2014).
Ye, J., Kalvet, I., Schoenebeck, F. & Rovis, T. Direct α-alkylation of primary aliphatic amines enabled by CO2 and electrostatics. Nat. Chem. 10, 1037–1041 (2018).
Png, Z. M., Cabrera-Pardo, J. R., Peiró Cadahía, J. & Gaunt, M. J. Diastereoselective C–H carbonylative annulation of aliphatic amines: a rapid route to functionalized γ-lactams. Chem. Sci. 9, 7628–7633 (2018).
Danishefsky, S., Berman, E., Clizbe, L. A. & Hirama, M. A simple synthesis of l-γ-carboxyglutamate and derivatives thereof. J. Am. Chem. Soc. 101, 4385–4386 (1979).
Seki, T., Tanaka, S. & Kitamura, M. Enantioselective synthesis of pyrrolidine-, piperidine-, and azepane-type N-heterocycles with α-alkenyl substitution: the CpRu-catalyzed dehydrative intramolecular N-allylation approach. Org. Lett. 14, 608–611 (2012).
Yuan, Q., Liu, D. & Zhang, W. Iridium-catalyzed asymmetric hydrogenation of β,γ-unsaturated γ-lactams: scope and mechanistic studies. Org. Lett. 19, 1144–1147 (2017).
Tahara, Y.-k, Michino, M., Ito, M., Kanyiva, K. S. & Shibata, T. Enantioselective sp 3 C–H alkylation of γ-butyrolactam by a chiral Ir(i) catalyst for the synthesis of 4-substituted γ-amino acids. Chem. Commun. 51, 16660–16663 (2015).
Gensch, T., Hopkinson, M. N., Glorius, F. & Wencel-Delord, J. Mild metal-catalyzed C–H activation: examples and concepts. Chem. Soc. Rev. 45, 2900–2936 (2016).
Davies, H. M. L. & Morton, D. Recent advances in C–H functionalization. J. Org. Chem. 81, 343–350 (2016).
Roizen, J. L., Harvey, M. E. & Du Bois, J. Metal-catalyzed nitrogen-atom transfer methods for the oxidation of aliphatic C–H bonds. Acc. Chem. Res. 45, 911–922 (2012).
Park, Y., Kim, Y. & Chang, S. Transition metal-catalyzed C–H amination: scope, mechanism, and applications. Chem. Rev. 117, 9247–9301 (2017).
Liang, J.-L., Yuan, S.-X., Huang, J.-S., Yu, W.-Y. & Che, C.-M. Highly diastereo- and enantioselective intramolecular amidation of saturated C–H bonds catalyzed by ruthenium porphyrins. Angew. Chem. Int. Ed. 41, 3465–3468 (2002).
Reddy, R. P. & Davies, H. M. L. Dirhodium tetracarboxylates derived from adamantylglycine as chiral catalysts for enantioselective C–H aminations. Org. Lett. 8, 5013–5016 (2006).
Zalatan, D. N. & Du Bois, J. A chiral rhodium carboxamidate catalyst for enantioselective C–H amination. J. Am. Chem. Soc. 130, 9220–9221 (2008).
Milczek, E., Boudet, N. & Blakey, S. Enantioselective C–H amination using cationic ruthenium(ii)–pybox catalysts. Angew. Chem. Int. Ed. 47, 6825–6828 (2008).
Ichinose, M. et al. Enantioselective intramolecular benzylic C–H bond amination: efficient synthesis of optically active benzosultams. Angew. Chem. Int. Ed. 50, 9884–9887 (2011).
McIntosh, J. A. et al. Enantioselective intramolecular C–H amination catalyzed by engineered cytochrome P450 enzymes in vitro and in vivo. Angew. Chem. Int. Ed. 52, 9309–9312 (2013).
Dydio, P., Key, H. M., Hayashi, H., Clark, D. S. & Hartwig, J. F. Chemoselective, enzymatic C–H bond amination catalyzed by a cytochrome P450 containing an Ir(Me)–PIX cofactor. J. Am. Chem. Soc. 139, 1750–1753 (2017).
Prier, C. K., Zhang, R. K., Buller, A. R., Brinkmann-Chen, S. & Arnold, F. H. Enantioselective, intermolecular benzylic C–H amination catalysed by an engineered iron-haem enzyme. Nat. Chem. 9, 629–634 (2017).
Li, C. et al. Catalytic radical process for enantioselective amination of C(sp 3)–H bonds. Angew. Chem. Int. Ed. 57, 16837–16841 (2018).
Liang, C. et al. Efficient diastereoselective intermolecular rhodium-catalyzed C–H amination. Angew. Chem. Int. Ed. 45, 4641–4644 (2006).
Nishioka, Y., Uchida, T. & Katsuki, T. Enantio- and regioselective intermolecular benzylic and allylic C–H bond amination. Angew. Chem. Int. Ed. 52, 1739–1742 (2013).
Pinho e Melo, T. N. V. D. in Organic Azides: Syntheses and Applications (eds Bräse, S. & Banert, K.) Ch. 3 (Wiley, New York, 2010).
Lebel, H. & Leogane, O. Boc-protected amines via a mild and efficient one-pot Curtius rearrangement. Org. Lett. 7, 4107–4110 (2005).
Li, D., Wu, T., Liang, K. & Xia, C. Curtius-like rearrangement of an iron–nitrenoid complex and application in biomimetic synthesis of bisindolylmethanes. Org. Lett. 18, 2228–2231 (2016).
Park, Y., Park, K. T., Kim, J. G. & Chang, S. Mechanistic studies on the Rh(iii)-mediated amido transfer process leading to robust C–H amination with a new type of amidating reagent. J. Am. Chem. Soc. 137, 4534–4542 (2015).
Park, Y., Jee, S., Kim, J. G. & Chang, S. Study of sustainability and scalability in the Cp*Rh(iii)-catalyzed direct C−H amidation with 1,4,2-dioxazol-5-ones. Org. Process Res. Dev. 19, 1024–1029 (2015).
Park, Y., Heo, J., Baik, M.-H. & Chang, S. Why is the Ir(iii)-mediated amido transfer much faster than the Rh(iii)-mediated reaction? A combined experimental and computational study. J. Am. Chem. Soc. 138, 14020–14029 (2016).
Hwang, Y., Park, Y. & Chang, S. Mechanism-driven approach to develop a mild and versatile C–H amidation through IrIII catalysis. Chem. Eur. J. 23, 11147–11152 (2017).
Hong, S. Y. et al. Selective formation of γ-lactams via C–H amidation enabled by tailored iridium catalysts. Science 359, 1016–1021 (2018).
Hennessy, E. T. & Betley, T. A. Complex N-heterocycle synthesis via iron-catalyzed, direct C–H bond amination. Science 340, 591–595 (2013).
Murata, K., Ikariya, T. & Noyori, R. New chiral rhodium and iridium complexes with chiral diamine ligands for asymmetric transfer hydrogenation of aromatic ketones. J. Org. Chem. 64, 2186–2187 (1999).
Camps, P. et al. Synthesis and absolute configuration of novel N,O-psiconucleosides using (R)-N-phenylpantolactam as a resolution agent. J. Org. Chem. 73, 6657–6665 (2008).
Heiden, Z. M., Gorecki, B. J. & Rauchfuss, T. B. Lewis base adducts derived from transfer hydrogenation catalysts: scope and selectivity. Organometallics 27, 1542–1549 (2008).
Koike, T. & Ikariya, T. Mechanistic aspects of formation of chiral ruthenium hydride complexes from 16‐electron ruthenium amide complexes and formic acid: facile reversible decarboxylation and carboxylation. Adv. Synth. Catal. 346, 37–41 (2004).
Muñiz, K. et al. Metal-ligand bifunctional activation and transfer of N−H bonds. Chem. Commun. 47, 4911–4913 (2011).
Jeffrey, G. A. & Takagi, S. Hydrogen-bond structure in carbohydrate crystals. Acc. Chem. Res. 11, 264–270 (1978).
Soni, R. et al. The importance of the N–H bond in Ru/TsDPEN complexes for asymmetric transfer hydrogenation of ketones and imines. Org. Biomol. Chem. 9, 3290–3294 (2011).
Flack, H. D. & Bernardinelli, G. The use of X-ray crystallography to determine absolute configuration. Chirality 20, 681–690 (2008).
Liang, J.-L., Yuan, S.-X., Huang, J.-S. & Che, C.-M. Intramolecular C−N bond formation reactions catalyzed by ruthenium porphyrins: amidation of sulfamate esters and aziridination of unsaturated sulfonamides. J. Org. Chem. 69, 3610–3619 (2004).
Saint-Denis, T. G., Zhu, R.-Y., Chen, G., Wu, Q.-F. & Yu, J.-Q. Enantioselective C(sp 3)‒H bond activation by chiral transition metal catalysts. Science 359, eaao4798 (2018).
Acknowledgements
This research was supported by the Institute for Basic Science (IBS-R010-D1) in Korea. The authors thank D. Kim (Institute for Basic Science) for X-ray analysis.
Author information
Authors and Affiliations
Contributions
Y.P. and S.C. conceived and designed the project and wrote the manuscript. Y.P. carried out the experiments and DFT calculations. S.C. organized the research. Both authors analysed the data, discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
Y.P. and S.C. are inventors on a patent application no. KR10–2018–0174064, submitted by IBS and KAIST, which covers the preparation and application of the related transition metal catalysts.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Figures 1–36, Supplementary Discussion, Supplementary Tables 1–5, Supplementary Notes 1–2, Supplementary References
Supplementary Data 1
Cartesian coordinates for the optimized structures
CCDC reference 1873623
Crystallographic Data for compound 6
CCDC reference 1873624
Crystallographic Data for compound 31
CCDC reference 1884674
Crystallographic Data for compound Ir10
CCDC reference 1873622
Crystallographic Data for compound Ir13
Rights and permissions
About this article
Cite this article
Park, Y., Chang, S. Asymmetric formation of γ-lactams via C–H amidation enabled by chiral hydrogen-bond-donor catalysts. Nat Catal 2, 219–227 (2019). https://doi.org/10.1038/s41929-019-0230-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-019-0230-x
This article is cited by
-
Stereoselective construction of β-, γ- and δ-lactam rings via enzymatic C–H amidation
Nature Catalysis (2023)
-
Cu-mediated enantioselective C–H alkynylation of ferrocenes with chiral BINOL ligands
Nature Communications (2023)
-
Ni-catalyzed carbamoylation of unactivated alkenes for stereoselective construction of six-membered lactams
Nature Communications (2022)
-
Stereocontrolled 1,3-nitrogen migration to access chiral α-amino acids
Nature Chemistry (2022)
-
C–H activation
Nature Reviews Methods Primers (2021)