Abstract
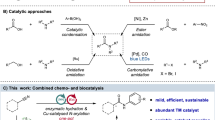
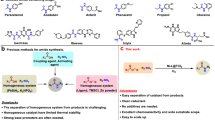
The synthesis of amides is of widespread importance, and there has been considerable recent interest in the development of catalytic methods to access these molecules. In this Perspective, we provide an overview of the current state of the art in amide synthesis, and assess new catalytic amide formation methods in the context of efficiency and sustainability. The advantages and disadvantages of catalytic approaches are highlighted and areas for future research are identified.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data analysed in this article are included in the Supplementary Information (SI) file.
References
Valeur, E. & Bradley, M. Amide bond formation: beyond the myth of coupling reagents. Chem. Soc. Rev. 38, 606–631 (2009).
Dunetz, J. R., Magano, J. & Weisenburger, G. A. Large-scale applications of amide coupling reagents for the synthesis of pharmaceuticals. Org. Process Res. Dev. 20, 140–177 (2016).
Anastas, P. T. & Warner, J. C. Green Chemistry: Theory and Practice (Oxford University Press, New York, 1998).
Jimenez-Gonzalez, C. et al. Using the right green yardstick: Why process mass intensity is used in the pharmaceutical industry to drive more sustainable processes. Org. Process Res. Dev. 15, 912–917 (2011).
Constable, D. J. C. et al. Key green chemistry research areas — a perspective from pharmaceutical manufacturers. Green Chem. 9, 411–420 (2007).
Brown, D. G. & Boström, J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem. 59, 4443–4458 (2016).
Sherwood, J., Farmer, T. J. & Clark, J. H. Catalyst: possible consequences of the N-methyl pyrrolidone REACH restriction. Chem. 4, 2010–2012 (2018).
Sherwood, J. European restrictions on 1,2-dichloroethane: C-H activation research and development should be liberated and not limited. Angew. Chem. Int. Ed. 57, 14286–14290 (2018).
Prat, D. et al. CHEM21 selection guide of classical- and less classical-solvents. Green Chem. 18, 288–296 (2016).
Li, C.-J. & Trost, B. M. Green chemistry for chemical synthesis. Proc. Natl Acad. Sci. USA 105, 13197–13202 (2008).
Ishihara, K., Ohara, S. & Yamamoto, H. 3,4,5-Trifluorobenzeneboronic acid as an extremely active amidation catalyst. J. Org. Chem. 61, 4196–4197 (1996).
MacMillan, D. S. et al. Evaluation of alternative solvents in common amide coupling reactions: replacement of dichloromethane and N,N-dimethylformamide. Green Chem. 15, 596–600 (2013).
Ho, G.-J. et al. Carbodiimide-mediated amide formation in a two-phase system. A high-yield and low-racemization procedure for peptide synthesis.J. Org. Chem. 60, 3569–3570 (1995).
Carey, J. S. et al. Analysis of the reactions used for the preparation of drug candidate molecules. Org. Biomol. Chem. 4, 2337–2347 (2006).
Braddock, D. C. et al. Tetramethyl orthosilicate (TMOS) as a reagent for direct amidation of carboxylic acids. Org. Lett. 20, 950–953 (2018).
Gabriel, C. M. et al. Amide and peptide bond formation in water at room temperature. Org. Lett. 17, 3968–3971 (2015).
Wehrstedt, K. D., Wandrey, P. A. & Heitkamp, D. Explosive properties of 1-hydroxybenzotriazoles. J. Hazard. Mater. 126, 1–7 (2005).
Allen, C. L., Chhatwal, A. R. & Williams, J. M. J. Direct amide formation from unactivated carboxylic acids and amines. Chem. Commun. 48, 666–668 (2012).
Lundberg, H., Tinnis, F. & Adolfsson, H. Direct amide coupling of non-activated carboxylic acids and amines catalysed by zirconium(IV) chloride. Chem. Eur. J. 18, 3822–3826 (2012).
Lundberg, H., Tinnis, F. & Adolfsson, H. Titanium(IV) isopropoxide as an efficient catalyst for direct amidation of nonactivated carboxylic acids. Synlett 23, 2201–2204 (2012).
Lundberg, H. & Adolfsson, H. Hafnium-catalyzed direct amide formation at room temperature. ACS Catal. 5, 3271–3277 (2015).
Tang, P. Boric acid catalyzed amide formation from carboxylic acids and amines: N-benzyl-4-phenylbutyramide. Org. Synth. 81, 262–272 (2005).
Sabatini, M. T., Boulton, L. T. & Sheppard, T. D. Borate esters: simple catalysts for the sustainable synthesis of complex amides. Sci Adv. 3, e1701028 (2017).
Sabatini, M. T. et al. Protecting-group-free amidation of amino acids using Lewis Acid catalysts. Chem. Eur. J. 24, 7033–7043 (2018).
Arnold, K. et al. Synthesis, evaluation and application of novel bifunctional N,N-diisopropylbenzylamineboronic acid catalysts for direct amide formation between carboxylic acids and amines. Green Chem. 10, 124–134 (2008).
Fatemi, S., Gernignon, N. & Hall, D. G. A multigram-scale lower E-factor procedure for MIBA-catalyzed direct amidation and its application to the coupling of alpha and beta aminoacids. Green Chem. 17, 4016–4028 (2015).
Wang, K., Lu, Y. & Ishihara, K. The ortho-substituent on 2,4-bis(trifluoromethyl)phenylboronic acid catalyzed dehydrative condensation between carboxylic acids and amines. Chem. Commun. 54, 5410–5413 (2018).
Maki, T., Ishihara, K. & Yamamoto, H. 4,5,6,7-Tetrachlorobenzo[d][1,3,2]dioxaborol-2-ol as an effective catalyst for the amide condensation of sterically demanding carboxylic acids. Org. Lett. 8, 1431–1434 (2006).
Noda, H. et al. Unique physicochemical and catalytic properties dictated by the B3NO2 ring system. Nat. Chem. 9, 571–577 (2017).
British Geological Survey http://www.go.nature.com/2PT10DY (2015).
Moore, J. A. et al. An assessment of boric acid and borax using the IEHR evaluative process for assessing human developmental and reproductive toxicity of agents. Reproductive Toxicol. 11, 123–160 (1997).
Bannister, R. B. et al. A scaleable route to the pure enantiomers of verapamil. Org. Process Res. Dev. 4, 467–472 (2000).
Mylavarapu, R. K. et al. Boric acid catalyzed amidation in the synthesis of active pharmaceutical ingredients. Org. Process Res. Dev. 11, 1065–1068 (2007).
Limanto, J. et al. A highly efficient asymmetric synthesis of vernakalant. Org. Lett. 16, 2716–2719 (2014).
Lee, D. S. et al. Investigating scale-up and further applications of DABAL-Me3 promoted amide synthesis. Org. Process Res. Dev. 19, 831–840 (2015).
Sabot, C. et al. A convenient aminolysis of esters catalyzed by 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) under solvent-free conditions. Tetrahedron Lett. 48, 3863–3866 (2007).
Weiberth, F. J. et al. Demonstration on pilot-plant scale of the utility of 1,5,7- triazabicyclo[4.4.0]dec-5-ene (TBD) as a catalyst in the efficient amidation of an unactivated methyl ester. Org. Process Res. Dev. 16, 1967–1969 (2012).
Yang, X. & Birman, V. B. Acyl transfer catalysis with 1,2,4-triazole anion. Org. Lett. 11, 1499–1502 (2009).
McPharson, C. G. et al. Amidation of unactivated ester derivatives mediated by trifluoroethanol. Org. Biomol. Chem. 15, 3507–3518 (2017).
Lenstra, D. C., Nguyen, D. T. & Mecinović, J. Zirconium-catalyzed direct amide bond formation between carboxylic esters and amines. Tetrahedron Lett. 71, 5547–5553 (2015).
Nguyen, D. T., Lenstra, D. C. & Mecinović, J. Chemoselective calcium-catalysed direct amidation of carboxylic esters. RSC Adv. 5, 77658–77661 (2015).
Morimoto, H. et al. Lanthanum(III) triflate catalyzed direct amidation of esters. Org. Lett. 16, 2018–2021 (2014).
Sanz Sharley, D. D. & Williams, J. M. J. Acetic acid as a catalyst for the N-acylation of amines using esters as the acyl source. Chem. Commun. 53, 2020–2023 (2017).
Tillack, A., Rudloff, I. & Beller, M. Catalytic amination of aldehydes to amides. Eur. J. Org. Chem. 2001, 523–.
Gunanathan, C., Ben-David, Y. & Milstein, D. Direct synthesis of amides from alcohols and amines with liberation of H2. Science 317, 790–792 (2007).
Fang, W. et al. Highly efficient aminocarbonylation of iodoarenes at atmospheric pressure catalyzed by a robust acenaphthoimidazolyidene allylic palladium complex. Org. Lett. 15, 3678–3681 (2013).
Dorr, B. M. & Fuerst, D. E. Enzymatic amidation for industrial applications. Curr. Opin. Chem. Bio. 43, 127–133 (2018).
Comerford, J. W. et al. Clean, reusable and low cost heterogeneous catalyst for amide synthesis. Chem. Commun. 0, 2562–2564 (2009).
Petchey, T. H. M. et al. Optimization of amidation reactions using predictive tools for the replacement of regulated solvents with safer biobased alternatives. ACS Sus. Chem. Eng. 6, 1550–1554 (2018).
Nadin, A., Hattotuwagama, C. & Churcher, I. Lead‐oriented synthesis: a new opportunity for synthetic chemistry. Angew. Chem. Int. Ed. 51, 1114–1122 (2012).
Alder, C. M. et al. Updating and further expanding GSK’s solvent sustainability guide.Green Chem. 18, 3879–3890 (2016).
Acknowledgements
M.T.S. would like to acknowledge financial support for a postdoctoral position from UCL via the EPSRC Impact Acceleration Account (EP/R511638/1), and for a PhD studentship from UCL and GSK. L.T.B and H.F.S would like to thank K. Wheelhouse (GSK) for initial work on GSK portfolio reaction classification.
Author information
Authors and Affiliations
Contributions
M.T.S. and T.D.S wrote the manuscript and collected and analysed the Reaxys dataset, L.T.B and H.F.S. collected and analysed the large-scale amidation dataset and contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Data 1
Data supporting Figures 2 and 3
Rights and permissions
About this article
Cite this article
Sabatini, M.T., Boulton, L.T., Sneddon, H.F. et al. A green chemistry perspective on catalytic amide bond formation. Nat Catal 2, 10–17 (2019). https://doi.org/10.1038/s41929-018-0211-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-018-0211-5
This article is cited by
-
Crosslinking Methods in Polysaccharide-Based Hydrogels for Drug Delivery Systems
Biomedical Materials & Devices (2024)
-
Oxidative cleavage and ammoxidation of organosulfur compounds via synergistic Co-Nx sites and Co nanoparticles catalysis
Nature Communications (2023)
-
Practical N-to-C peptide synthesis with minimal protecting groups
Communications Chemistry (2023)
-
Efficient electrosynthesis of formamide from carbon monoxide and nitrite on a Ru-dispersed Cu nanocluster catalyst
Nature Communications (2023)
-
Defect engineering of electrocatalysts for organic synthesis
Nano Research (2023)