Abstract
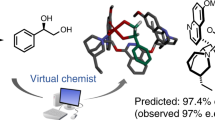
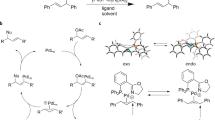
The development of computational tools to support organic synthesis, including the prediction of reaction pathways, optimization and selectivity, is a topic of intense current interest. Transition state force fields, derived by the quantum-guided molecular mechanics method, rapidly calculate the stereoselectivity of organic reactions accurately enough to allow predictive virtual screening. Here we describe CatVS, an automated tool for the virtual screening of substrate and ligand libraries for asymmetric catalysis within hours. It is shown for the OsO4-catalysed cis-dihydroxylation that the results from the automated set-up are indistinguishable from a manual substrate screen. Predictive computational ligand selection is demonstrated in the virtual ligand screen of a library of diphosphine ligands for the rhodium-catalysed asymmetric hydrogenation of enamides. Subsequent experimental testing verified that the most selective substrate–ligand combinations are successfully identified by the virtual screen. CatVS is therefore a promising tool to increase the efficiency of high-throughput experimentation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All other data is available from the authors upon reasonable request.
References
Kitchen, D. B., Decornez, H., Fur, J. R. & Bajorath, J. Docking and scoring in virtual screening for drug discovery: methods and applications. Nat. Rev. Drug Discov. 3, 935–949 (2004).
Klucznik, T. et al. Efficient syntheses of diverse, medicinally relevant targets planned by computer and executed in the laboratory. Chem 4, 522–532 (2018).
Segler, M. H. S., Preuss, M. & Waller, M. P. Planning chemical syntheses with deep neural networks and symbolic AI. Nature 555, 604–610 (2018).
Ahneman, D. T., Estrada, J. G., Lin, S., Dreher, S. D. & Doyle, A. G. Predicting reaction performance in C–N cross-coupling using machine learning. Science 360, 186–190 (2018).
Poree, C. & Schoenebeck, F. A. Holy grail in chemistry: computational catalyst design: feasible or fiction?. Acc. Chem. Res. 50, 605–608 (2017).
Shevlin, R. Practical high-throughput experimentation for chemists. ACS Med. Chem. Lett. 8, 601–607 (2017).
Houk, K. N. & Cheong, P. H. Y. Computational prediction of small-molecule catalyst. Nature 455, 309–313 (2008).
Kwon, D.-H. et al. Computational transition-state design provides experimentally verified Cr(P,N) catalysts for control of ethylene trimerization and tetramerization. ACS Catal. 8, 1138–1142 (2018).
Rooks, B. J., Haas, M. R., Sepúlveda, D., Lu, T. & Wheeler, S. E. Prospects for the computational design of bipyridine N,N′-dioxide catalysts for asymmetric propargylation reactions. ACS Catal. 5, 272–280 (2015).
Harper, K. C. & Sigman, M. S. Three-dimensional correlation of steric and electronic free energy relationships guides asymmetric propargylation. Science 333, 1875–1878 (2011).
Harper, K. C. & Sigman, M. S. Predicting and optimizing asymmetric catalyst performance using the principles of experimental design and steric parameters. Proc. Natl Acad. Sci. USA 108, 2179–2183 (2011).
Milo, A., Neel, A. J., Toste, F. D. & Sigman, M. S. A data-intensive approach to mechanistic elucidation applied to chiral anion catalysis. Science 347, 737–743 (2015).
Tropsha, A. Best practices for QSAR model development, validation, and exploitation. Mol. Inf. 29, 476–488 (2010).
Hansen, E., Rosales, A. R., Tutkowski, B., Norrby, P.-O. & Wiest, O. Prediction of stereochemistry using Q2MM. Acc. Chem. Res. 49, 996–1005 (2016).
Rosales, A. R. et al. Application of Q2MM to predictions in stereoselective synthesis. Chem. Comm. 54, 8294–8311 (2018).
Eksterowicz, J. E. & Houk, K. N. Transition-state modeling with empirical force fields. Chem. Rev. 93, 2439–2461 (1993).
Norrby, P.-O., Rasmussen, T., Haller, J., Strassner, T. & Houk, K. N. Rationalizing the stereoselectivity of osmium tetroxide asymmetric dihydroxylations with transition state modeling using quantum mechanics-guided molecular mechanics. J. Am. Chem. Soc. 121, 10186–10192 (1999).
Fristrup, P., Tanner, D. & Norrby, P.-O. Updating the asymmetric osmium‐catalyzed dihydroxylation (AD) mnemonic: Q2MM modeling and new kinetic measurements. Chirality 15, 360–368 (2003).
Fristrup, P., Jensen, G. H., Andersen, M. L. N., Tanner, D. & Norrby, P.-O. Combining Q2MM modeling and kinetic studies for refinement of the osmium-catalyzed asymmetric dihydroxylation (AD) mnemonic. J. Organomet. Chem. 691, 2182–2198 (2006).
Donoghue, P. J., Helquist, P., Norrby, P.-O. & Wiest, O. Development of a Q2MM force field for the asymmetric rhodium catalyzed hydrogenation of enamides. J. Chem. Theory Comput. 4, 1313–1323 (2008).
Donoghue, P. J., Helquist, P., Norrby, P.-O. & Wiest, O. Prediction of enantioselectivity in rhodium catalyzed hydrogenations. J. Am. Chem. Soc. 131, 410–411 (2009).
Lime, E. et al. Stereoselectivity in asymmetric catalysis: the case of ruthenium-catalyzed ketone hydrogenation. J. Chem. Theory Comput. 10, 2427–2435 (2014).
Le, D. N. et al. Hydrogenation catalyst generates cyclic peptide stereocentres in sequence. Nat. Chem. 10, 968–973 (2018).
Norrby, P.-O., Brandt, P. & Rein, T. Rationalization of product selectivities in asymmetric Horner–Wadsworth–Emmons reactions by use of a new method for transition-state modeling. J. Org. Chem. 64, 5845–5852 (1999).
Rasmussen, T. & Norrby, P.-O. Modeling the stereoselectivity of the β-amino alcohol promoted addition of dialkylzinc to aldehydes. J. Am. Chem. Soc. 125, 5130–5138 (2003).
Lee, J. M. et al. Stereoselectivity in (acylox)borane-catalyzed Mukaiyama aldol reactions. J. Org. Chem. 81, 5314–5321 (2016).
Rydberg, P. et al. Transition-state docking of flunitrazepam and progesterone in cytochrome P450. J. Chem. Theory Comput. 4, 673–681 (2008).
Brown, D. G. & Boström, J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem. 59, 4443–4458 (2016).
Allinger, N. L., Yuh, Y. H. & Lii, J.-H. Molecular mechanics. The MM3 force field for hydrocarbons. 1. J. Am. Chem. Soc. 111, 8551–8566 (1989).
Schrödinger Suite v.2017-2 (Schrödinger, 2017).
Chang, G., Guida, W. C. & Still, W. C. An internal-coordinate Monte Carlo method for searching conformational space. J. Am. Chem. Soc. 111, 4379–4386 (1989).
Kolossváry, I. & Guida, W. C. Low mode search. An efficient, automated computational method for conformational analysis: application to cyclic and acyclic alkanes and cyclic peptides. J. Am. Chem. Soc. 118, 5011–5019 (1996).
Scior, T. et al. Recognizing pitfalls in virtual screening: a critical review. J. Chem. Inf. Mod. 52, 867–881 (2012).
Warren, G. L. et al. A critical assessment of docking programs and scoring functions. J. Med. Chem. 49, 5912–5931 (2006).
Acknowledgements
This work was supported financially by NSF (CHE-1565669), NIH (T32 GM075762 and 1R01GM111645) and AstraZeneca. S. Tomasi, D. Buttar, and J. Westin at AstraZeneca are acknowledged for help with the CatVS web implementation.
Author information
Authors and Affiliations
Contributions
E.H. and A.R.R. wrote the code, J.W. and E.L. performed calculations, R.H.M., R.M., K.W.L., R.S. and F.B. performed experiments. All authors designed the study, analyzed the data and contributed to the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Figures 1–5, Supplementary Tables 1–4, Supplementary References
Rights and permissions
About this article
Cite this article
Rosales, A.R., Wahlers, J., Limé, E. et al. Rapid virtual screening of enantioselective catalysts using CatVS. Nat Catal 2, 41–45 (2019). https://doi.org/10.1038/s41929-018-0193-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-018-0193-3
This article is cited by
-
Organic reactivity from mechanism to machine learning
Nature Reviews Chemistry (2021)
-
Proofreading experimentally assigned stereochemistry through Q2MM predictions in Pd-catalyzed allylic aminations
Nature Communications (2021)
-
Optical deciphering of multinary chiral compound mixtures through organic reaction based chemometric chirality sensing
Nature Communications (2021)
-
Automation and computer-assisted planning for chemical synthesis
Nature Reviews Methods Primers (2021)
-
From desktop to benchtop with automated computational workflows for computer-aided design in asymmetric catalysis
Nature Catalysis (2020)