Abstract
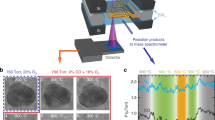
NiFeOxHy are the most active catalysts for oxygen evolution in a base. For this reason, they are used widely in alkaline electrolysers. Several open questions remain as to the reason for their exceptionally high catalytic activity. Here we use a model system of mass-selected NiFe nanoparticles and isotope labelling experiments to show that oxygen evolution in 1 M KOH does not proceed via lattice exchange. We complement our activity measurements with electrochemistry–mass spectrometry, taken under operando conditions, and transmission electron microscopy and low-energy ion-scattering spectroscopy, taken ex situ. Together with the trends in particle size, the isotope results indicate that oxygen evolution is limited to the near-surface region. Using the surface area of the particles, we determined that the turnover frequency was 6.2 ± 1.6 s−1 at an overpotential of 0.3 V, which is, to the best of our knowledge, the highest reported for oxygen evolution in alkaline solution.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.
References
Lewis, N. S. & Nocera, D. G. Powering the planet: chemical challenges in solar energy utilization. Proc. Natl Acad. Sci. USA 103, 15729–15735 (2006).
Debe, M. K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 486, 43–51 (2012).
Montoya, J. H. et al. Materials for solar fuels and chemicals. Nat. Mater. 16, 70–81 (2017).
Hong, W. T. et al. Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis. Energy Environ. Sci. 8, 1404–1427 (2015).
Durst, J. et al. New insights into the electrochemical hydrogen oxidation and evolution reaction mechanism. Energy Environ. Sci. 7, 2255–2260 (2014).
Stamenkovic, V. R., Strmcnik, D., Lopes, P. P. & Markovic, N. M. Energy and fuels from electrochemical interfaces. Nat. Mater. 16, 57–69 (2017).
Greeley, J. & Markovic, N. M. The road from animal electricity to green energy: combining experiment and theory in electrocatalysis. Energy Environ. Sci. 5, 9246 (2012).
Cifrain, M. & Kordesch, K. in Handbook of Fuel Cells—Fundamentals, Technology and Applications Vol. 1 (eds Vielstich, W.A., Gasteiger, H.A. & Lamm, A.) 267–280 (Wiley, Hoboken, 2010).
Pagliaro, M., Konstandopoulos, A. G., Ciriminna, R. & Palmisano, G. Solar hydrogen: fuel of the near future. Energy Environ. Sci. 3, 279 (2010).
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017).
The production of hydrogen and oxygen through the electrolysis of water. Scientific American Supplement No. 819 Vol. XXXII, article II (1891).
Trotochaud, L., Young, S. L., Ranney, J. K. & Boettcher, S. W. Nickel–iron oxyhydroxide oxygen-evolution electrocatalysts: the role of intentional and incidental iron incorporation. J. Am. Chem. Soc. 136, 6744–6753 (2014).
Dionigi, F. & Strasser, P. NiFe-based (oxy)hydroxide catalysts for oxygen evolution reaction in non-acidic electrolytes. Adv. Energy Mater. 6, 1600621 (2016).
Burke, M. S., Enman, L. J., Batchellor, A. S., Zou, S. & Boettcher, S. W. Oxygen evolution reaction electrocatalysis on transition metal oxides and (oxy)hydroxides: activity trends and design principles. Chem. Mater. 27, 7549–7558 (2015).
Enman, L. J., Burke, M. S., Batchellor, A. S. & Boettcher, S. W. Effects of intentionally incorporated metal cations on the oxygen evolution electrocatalytic activity of nickel (oxy)hydroxide in alkaline media. ACS Catal. 6, 2416–2423 (2016).
Klaus, S., Cai, Y., Louie, M. W., Trotochaud, L. & Bell, A. T. Effects of Fe electrolyte impurities on Ni(OH)2 /NiOOH structure and oxygen evolution activity. J. Phys. Chem. C 119, 7243–7254 (2015).
Li, N. et al. Influence of iron doping on tetravalent nickel content in catalytic oxygen evolving films. Proc. Natl Acad. Sci. USA 114, 1486–1491 (2017).
Görlin, M. et al. Oxygen evolution reaction dynamics, Faradaic charge efficiency, and the active metal redox states of Ni–Fe oxide water splitting electrocatalysts. J. Am. Chem. Soc. 138, 5603–5614 (2016).
Friebel, D. et al. Identification of highly active Fe sites in (Ni,Fe)OOH for electrocatalytic water splitting. J. Am. Chem. Soc. 137, 1305–1313 (2015).
Stevens, M. B., Trang, C. D. M., Enman, L. J., Deng, J. & Boettcher, S. W. Reactive Fe-Sites in Ni/Fe (oxy)hydroxide are responsible for exceptional oxygen electrocatalysis activity. J. Am. Chem. Soc. 139, 11361–11364 (2017).
Hunter, B., Winkler, J. & Gray, H. Iron is the active site in nickel/iron water oxidation electrocatalysts. Molecules 23, 903 (2018).
Ahn, H. S. & Bard, A. J. Surface interrogation scanning electrochemical microscopy of Ni1–xFexOOH (0 < x < 0.27) oxygen evolving catalyst: kinetics of the ‘fast’ iron sites. J. Am. Chem. Soc. 138, 313–318 (2016).
Xiao, H., Shin, H. & Goddard, W. A. Synergy between Fe and Ni in the optimal performance of (Ni,Fe)OOH catalysts for the oxygen evolution reaction. Proc. Natl Acad. Sci. USA 115, 5872–5877 (2018).
Batchellor, A. S. & Boettcher, S. W. Pulse-electrodeposited Ni–Fe (oxy)hydroxide oxygen evolution electrocatalysts with high geometric and intrinsic activities at large mass loadings. ACS Catal. 5, 6680–6689 (2015).
Doyle, A. D., Bajdich, M. & Vojvodic, A. Theoretical insights to bulk activity towards oxygen evolution in oxyhydroxides. Catal. Lett. 147, 1533–1539 (2017).
Morales-Guio, C. G., Liardet, L. & Hu, X. Oxidatively electrodeposited thin-film transition metal (oxy)hydroxides as oxygen evolution catalysts. J. Am. Chem. Soc. 138, 8946–8957 (2016).
Trotochaud, L., Ranney, J. K., Williams, K. N. & Boettcher, S. W. Solution-cast metal oxide thin film electrocatalysts for oxygen evolution. J. Am. Chem. Soc. 134, 17253–17261 (2012).
Wohlfahrt-Mehrens, M. & Heitbaum, J. Oxygen evolution on Ru and RuO2 electrodes studied using isotope labelling and on-line mass spectrometry. J. Electroanal. Chem. Interfacial Electrochem. 237, 251–260 (1987).
Diaz-Morales, O., Calle-Vallejo, F., de Munck, C. & Koper, M. T. M. Electrochemical water splitting by gold: evidence for an oxide decomposition mechanism. Chem. Sci. 4, 2334 (2013).
Fierro, S., Nagel, T., Baltruschat, H. & Comninellis, C. Investigation of the oxygen evolution reaction on Ti/IrO2 electrodes using isotope labelling and on-line mass spectrometry. Electrochem. Commun. 9, 1969–1974 (2007).
Amin, H. M. A. & Baltruschat, H. How many surface atoms in Co3O4 take part in oxygen evolution? Isotope labeling together with differential electrochemical mass spectrometry. Phys. Chem. Chem. Phys. 19, 25527–25536 (2017).
Surendranath, Y., Kanan, M. W. & Nocera, D. G. Mechanistic studies of the oxygen evolution reaction by a cobalt–phosphate catalyst at neutral pH. J. Am. Chem. Soc. 132, 16501–16509 (2010).
Macounova, K., Makarova, M. & Krtil, P. Oxygen evolution on nanocrystalline RuO2 and Ru0.9Ni0.1O2−δ electrodes—DEMS approach to reaction mechanism determination. Electrochem. Commun. 11, 1865–1868 (2009).
Grimaud, A. et al. Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution. Nat. Chem. 9, 457–465 (2017).
Willsau, J., Wolter, O. & Heitbaum, J. In the oxygen evolution reaction on platinum? J. Electroanal. Chem. 195, 299–306 (1985).
Stoerzinger, K. A. et al. Orientation-dependent oxygen evolution on RuO2 without lattice exchange. ACS Energy Lett. 2, 876–881 (2017).
Lu, X. & Zhao, C. Electrodeposition of hierarchically structured three-dimensional nickel–iron electrodes for efficient oxygen evolution at high current densities. Nat. Commun. 6, 6616 (2015).
Trimarco, D. B. et al. Enabling real-time detection of electrochemical desorption phenomena with sub-monolayer sensitivity. Electrochim. Acta 268, 520–530 (2018).
Paoli, E. A. et al. Oxygen evolution on well-characterized mass-selected Ru and RuO2 nanoparticles. Chem. Sci. 6, 190–196 (2015).
Li, Z. Y. et al. Three-dimensional atomic-scale structure of size-selected gold nanoclusters. Nature 451, 46–48 (2008).
Ferreira, P. J. et al. Instability of Pt∕C electrocatalysts in proton exchange membrane fuel cells. J. Electrochem. Soc. 152, A2256 (2005).
Meier, J. C. et al. Design criteria for stable Pt/C fuel cell catalysts. Beilstein J. Nanotechnol. 5, 44–67 (2014).
Fiordaliso, E. M., Dahl, S. & Chorkendorff, I. Strong metal support interaction of Pt and Ru nanoparticles deposited on HOPG probed by the H–D exchange reaction. J. Phys. Chem. C 116, 5773–5780 (2012).
Binninger, T. et al. Thermodynamic explanation of the universal correlation between oxygen evolution activity and corrosion of oxide catalysts. Sci. Rep. 5, 12167 (2015).
Spöri, C., Kwan, J. T. H., Bonakdarpour, A., Wilkinson, D. P. & Strasser, P. The stability challenges of oxygen evolving catalysts: towards a common fundamental understanding and mitigation of catalyst degradation. Angew. Chem. Int. Ed. 56, 5994–6021 (2017).
Hartl, K., Hanzlik, M. & Arenz, M. IL-TEM investigations on the degradation mechanism of Pt/C electrocatalysts with different carbon supports. Energy Environ. Sci. 4, 234–238 (2011).
Ng, J. W. D. et al. Gold-supported cerium-doped NiOx catalysts for water oxidation. Nat. Energy 1, 16053 (2016).
Xu, X., Song, F. & Hu, X. A nickel iron diselenide-derived efficient oxygen-evolution catalyst. Nat. Commun. 7, 12324 (2016).
Silva, H. et al. Synthesis and characterization of Fe-Ni/χ-Al2O3 egg-shell catalyst for H2 generation by ammonia decomposition. Appl. Catal. A 505, 548–556 (2015).
Burke, M. S. et al. Revised oxygen evolution reaction activity trends for first-row transition-metal (oxy)hydroxides in alkaline media. J. Phys. Chem. Lett. 6, 3737–3742 (2015).
Zhang, B. et al. Homogeneously dispersed multimetal oxygen-evolving catalysts. Science 352, 333–337 (2016).
Gong, M. et al. An advanced Ni–Fe layered double hydroxide electrocatalyst for water oxidation. J. Am. Chem. Soc. 135, 8452–8455 (2013).
Song, F. & Hu, X. Exfoliation of layered double hydroxides for enhanced oxygen evolution catalysis. Nat. Commun. 5, 4477 (2014).
Long, X. et al. A strongly coupled graphene and FeNi double hydroxide hybrid as an excellent electrocatalyst for the oxygen evolution reaction. Angew. Chem. Int. Ed. 53, 7584–7588 (2014).
Wehrens-Dijksma, M. & Notten, P. H. L. Electrochemical quartz microbalance characterization of Ni(OH)2-based thin film electrodes. Electrochim. Acta 51, 3609–3621 (2006).
Lu, X. & Zhao, C. Electrodeposition of hierarchically structured densities. Nat. Commun. 6, 1–7 (2015).
von Issendorff, B. & Palmer, R. E. A new high transmission infinite range mass selector for cluster and nanoparticle beams. Rev. Sci. Instrum. 70, 4497–4501 (1999).
Pratontep, S., Carroll, S. J., Xirouchaki, C., Streun, M. & Palmer, R. E. Size-selected cluster beam source based on radio frequency magnetron plasma sputtering and gas condensation. Rev. Sci. Instrum. 76, 045103 (2005).
Biesinger, M. C., Lau, L. W. M., Gerson, A. R. & Smart, R. S. C. The role of the Auger parameter in XPS studies of nickel metal, halides and oxides. Phys. Chem. Chem. Phys. 14, 2434 (2012).
Biesinger, M. C. et al. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 257, 2717–2730 (2011).
Grosvenor, A. P., Biesinger, M. C., Smart, R. S. C. & McIntyre, N. S. New interpretations of XPS spectra of nickel metal and oxides. Surf. Sci. 600, 1771–1779 (2006).
Acknowledgements
This work was supported by a research grant (9455) from VILLUM FONDEN. We also acknowledge UPCAT under project no. 2015-1-12315.
Author information
Authors and Affiliations
Contributions
I.E.L.S., J.K. and I.C. conceived the experiments. C.R. participated in the conception of the experiments and performed the electrochemical measurements. B.S. participated in the conception of the experiments, prepared the nanoparticles and performed the UHV experiments. S.B.S. performed and helped in the design of the EC–MS experiments. D.B.T., P.C.K.V. and O.H. designed and helped with the interpretation of the EC–MS experiments. E.M.F. and C.D.D. performed the microscopy characterization. J.E.S. and A.B. contributed to the LEIS measurements. C.R., B.S. and S.B.S. co-wrote the manuscript. All the authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–8, Supplementary Figures 1–17 and Supplementary References
Rights and permissions
About this article
Cite this article
Roy, C., Sebok, B., Scott, S.B. et al. Impact of nanoparticle size and lattice oxygen on water oxidation on NiFeOxHy. Nat Catal 1, 820–829 (2018). https://doi.org/10.1038/s41929-018-0162-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-018-0162-x
This article is cited by
-
Cooperative Fe sites on transition metal (oxy)hydroxides drive high oxygen evolution activity in base
Nature Communications (2023)
-
Active oxygen species mediate the iron-promoting electrocatalysis of oxygen evolution reaction on metal oxyhydroxides
Nature Communications (2023)
-
Grand Canonical Quantum Mechanics with Applications to Mechanisms and Rates for Electrocatalysis
Topics in Catalysis (2023)
-
Encapsulated Bio-insecticide from Citrus aurantium (Rutaceae) Essential Oil and Pectin and Potential for the Control of the Lesser Grain Borer Rhyzopertha dominica (Bostrichidae)
Waste and Biomass Valorization (2023)
-
Disordered Rocksalts with Lattice Oxygen Activation as Efficient Oxygen Evolution Electrocatalysts
Transactions of Tianjin University (2023)