Abstract
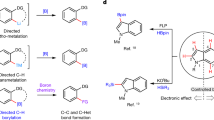
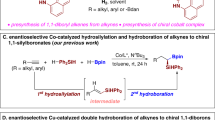
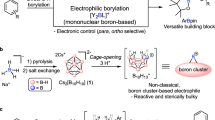
Multi-borylated compounds are useful starting materials for the construction of complex molecules. Although certain classes of multi-borylated compounds, such as geminal and 1,2-bis(boronates), can now be accessed selectively by several well-established methods, the synthesis of one class—those containing more than two boronate substituents—remains a great challenge. Here, copper catalytic systems were developed for the borylation of gem-difluoroalkenes with B2pin2 via dual C–F bond activation to afford multi-borylate libraries—1,2-alkyldiboronates, 1,1,2-alkyltriboronates and 1,1,1,2-alkyltetraboronates—by slightly tuning the reaction conditions. The advantages of this strategy include not only avoiding the use of different methods and substrates for each type of multi-substituted alkyl boronate, but also the excellent functional group compatibility, readily accessible gem-difluorovinyl group and highly chemoselective process.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Crystallographic data for the structures 5f and 6f reported in this paper have been deposited at the Cambridge Crystallographic Data Centre under deposition numbers 1855882 and 1855883. Copies of the data can be obtained free of charge via www.ccdc.cam.ac.uk/getstructures. All other data supporting the findings of this study, including experimental procedures and compound characterization, are available within the paper and its Supplementary Information, or from the corresponding author upon reasonable request.
References
Hall, D. G. Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials Vol. 1–2 (Wiley, Weinheim, 2011).
Brooks, W. L. A. & Sumerlin, B. S. Synthesis and applications of boronic acid-containing polymers: from materials to medicine. Chem. Rev. 116, 1375–1397 (2016).
Trippier, P. C. & McGuigan, C. Boronic acids in medicinal chemistry: anticancer, antibacterial and antiviral applications. Med. Chem. Comm. 1, 183–198 (2010).
Draganov, A., Wang, D. & Wang, B. in Atypical Elements in Drug Design (ed. Schwarz, J.) 1–27 (Springer, Basel, 2014).
Smoum, R., Rubinstein, A., Dembitsky, V. M. & Srebnik, M. Boron containing compounds as protease inhibitors. Chem. Rev. 112, 4156–4220 (2012).
Miyaura, N. & Yamamoto, Y. in Comprehensive Organometallic Chemistry III Vol. 9 (eds Crabtree, R. H. & Mingos, D. M. P.) Ch. 5 (Elsevier, New York, 2007).
Suzuki, A. Cross-coupling reactions of organoboranes: an easy way to construct C–C bonds (Nobel Lecture). Angew. Chem. Int. Ed. 50, 6722–6737 (2011).
Brown, H. C. Hydroboration (Benjamin/Cummings, San Francisco, 1980).
Crudden, C. M. & Edwards, D. Catalytic asymmetric hydroboration: recent advances and applications in carbon–carbon bond-forming reactions. Eur. J. Org. Chem. 2003 4695–4712 (2003).
Xu, L., Zhang, S. & Li, P. Boron-selective reactions as powerful tools for modular synthesis of diverse complex molecules. Chem. Soc. Rev. 44, 8848–8858 (2015).
Neeve, E. C., Geier, S. J., Mkhalid, I. A. I., Westcott, S. A. & Marder., T. B. Diboron(4) compounds: from structural curiosity to synthetic workhorse. Chem. Rev. 116, 9091–9161 (2016).
Issaian, A., Tu, K. N. & Blum, S. A. Boron–heteroatom addition reactions via borylative heterocyclization: oxyboration, aminoboration, and thioboration. Acc. Chem. Res. 50, 2598–2609 (2017).
Endo, K., Ohkubo, T., Hirokami, M. & Shibata, T. Chemoselective and regiospecific Suzuki coupling on a multisubstituted sp3-carbon in 1,1-diborylalkanes at room temperature. J. Am. Chem. Soc. 132, 11033–11035 (2010).
Zhang, Z.-Q. et al. Copper-catalyzed/promoted cross-coupling of gem-diborylalkanes with nonactivated primary alkyl halides: an alternative route to alkylboronic esters. Org. Lett. 16, 6342–6345 (2014).
Sun, C., Potter, B. & Morken, J. P. A catalytic enantiotopic-group-selective Suzuki reaction for the construction of chiral organoboronates. J. Am. Chem. Soc. 136, 6534–6537 (2014).
Bose, S. K. et al. Highly efficient synthesis of alkylboronate esters via Cu(ii)-catalysed borylation of unactivated alkyl bromides and chlorides in air. ACS Catal. 6, 8332–8335 (2016).
Jo, W., Kim, J., Choi, S. & Cho, S. H. Transition-metal-free regioselective alkylation of pyridine N-oxides using 1,1-diborylalkanes as alkylating reagents. Angew. Chem. Int. Ed. 55, 9690–9694 (2016).
Miralles, N., Gómez, J. E., Kleij, A. W. & Fernández, E. Copper-mediated SN2′ allyl–alkyl and allyl–boryl couplings of vinyl cyclic carbonates. Org. Lett. 19, 6096–6099 (2017).
Toribatake, K. & Nishiyama, H. Asymmetric diboration of terminal alkenes with a rhodium catalyst and subsequent oxidation: enantioselective synthesis of optically active 1,2-diols. Angew. Chem. Int. Ed. 52, 11011–11015 (2013).
Mlynarski, S. N., Schuster, C. H. & Morken, J. P. Asymmetric synthesis from terminal alkenes by cascades of diboration and cross-coupling. Nature 505, 386–390 (2014).
Blaisdell, T. P., Caya, T. C., Zhang, L., Sanz-Marco, A. & Morken, J. P. Hydroxyl-directed stereoselective diboration of alkenes. J. Am. Chem. Soc. 136, 9264–9267 (2014).
Crudden, C. M. et al. Iterative protecting group-free cross-coupling leading to chiral multiply arylated structures. Nat. Commun. 7, 11065–11071 (2016).
Fang, L., Yan, L., Haeffner, F. & Morken, J. P. Carbohydrate-catalysed enantioselective alkene diboration: enhanced reactivity of 1,2-bonded diboron complexes. J. Am. Chem. Soc. 138, 2508–2511 (2016).
Fawcett, A. et al. Regio- and stereoselective homologation of 1,2-bis(boronic esters): stereocontrolled synthesis of 1,3-diols and Sch 725674. Angew. Chem. Int. Ed. 55, 14883–14887 (2016).
Marder, T. B. & Norman, N. C. Transition metal catalysed diboration. Top. Catal. 5, 63–73 (1998).
Ramirez, J., Lillo, V., Segarra, A. M. & Fernández, E. Catalytic asymmetric boron–boron addition to unsaturated molecules. C. R. Chim. 10, 138–151 (2007).
Burks, H. E. & Morken, J. P. Catalytic enantioselective diboration, disilation and silaboration: new opportunities for asymmetric synthesis. Chem. Commun. 2007, 4717–4725 (2007).
Takaya, J. & Iwasawa, N. Catalytic, direct synthesis of bis(boronate) compounds. ACS Catal. 2, 1993–2006 (2012).
Cuenca, A. B., Shishido, R., Ito, H. & Fernández, E. Transition-metal-free B–B and B–interelement reactions with organic molecules. Chem. Soc. Rev. 46, 415–430 (2017).
Nallagonda, R., Padalaa, K. & Masarwa, A. gem-Diborylalkanes: recent advances in their preparation, transformation and application. Org. Biomol. Chem. 16, 1050–1064 (2018).
Miralles, N., Maza, R. J. & Fernándeza, E. Synthesis and reactivity of 1,1-diborylalkanes towards C–C bond formation and related mechanisms. Adv. Synth. Catal. 360, 1306–1327 (2018).
Gu, Y., Pritzkow, H. & Siebert, W. Synthesis and reactivity of monoborylacetylene derivatives. Eur. J. Inorg. Chem. 2001, 373–379 (2001).
Baker, R. T., Nguyen, P., Marder, T. B. & Westcott, S. A. Transition metal catalysed diboration of vinylarenes. Angew. Chem. Int. Ed. 34, 1336–1338 (1995).
Nguyen, P. et al. Rhodium(i) catalysed diboration of (E)-styrylboronate esters: molecular structures of (E)-p-MeO–C6H4–CH:CH–B(1,2-O2C6H4) and p-MeO–C6H4–CH2C{B(1,2-O2C6H4)}3. J. Organomet. Chem. 652, 77–85 (2002).
Huang, Z. & Zhang, L. Synthesis of 1,1,1-tris(boronates) from vinylarenes by co-catalyzed dehydrogenative borylations–hydroboration. J. Am. Chem. Soc. 137, 15600–15603 (2015).
Shimada, S., Batsanov, A. S., Howard, J. A. K. & Marder, T. B. Formation of aryl- and benzylboronate esters by rhodium-catalyzed C–H bond functionalization with pinacolborane. Angew. Chem. Int. Ed. 40, 2168–2171 (2001).
Cho, J. Y. et al. Remarkably selective iridium catalysts for the elaboration of aromatic C–H bonds. Science 295, 305–308 (2002).
Hartwig, J. F. Regioselectivity of the borylation of alkanes and arenes. Chem. Soc. Rev. 40, 1992–2002 (2011).
Hartwig, J. F. Borylation and silylation of C–H bonds: a platform for diverse C–H bond functionalizations. Acc. Chem. Res. 45, 864–873 (2012).
Bose, S. K. & Marder, T. B. A leap forward in C–H functionalization. Science 349, 473–474 (2015).
Mita, T., Ikeda, Y., Michigami, K. & Sato, Y. Iridium-catalysed triple C(sp3)–H borylations: construction of triborylated sp3-carbon centers. Chem. Commun. 49, 5601–5603 (2013).
Palmer, W. N., Obligacion, J. V., Pappas, I. & Chirik, P. J. Cobalt-catalysed benzylic borylation: enabling polyborylation and functionalization of remote, unactivated C(sp3)–H bonds. J. Am. Chem. Soc. 138, 766–769 (2016).
Krautwald, S., Bezdek, M. J. & Chirik, P. J. Cobalt-catalyzed 1,1-diboration of terminal alkynes: scope, mechanism and synthetic applications. J. Am. Chem. Soc. 139, 3868–3875 (2017).
Coombs, J. R., Zhang, L. & Morken, J. P. Enantiomerically enriched tris(boronates): readily accessible conjunctive reagents for asymmetric synthesis. J. Am. Chem. Soc. 136, 16140–16143 (2014).
Gao, G., Yan, J., Yang, K., Chen, F. & Song, Q. Base-controlled highly selective synthesis of alkyl 1,2-bis(boronates) or 1,1,2-tris(boronates) from terminal alkynes. Green Chem. 19, 3997–4001 (2017).
Castle, R. B. & Matteson, D. S. Methanetetraboroni and methanetriboronic esters. J. Organometal. Chem. 20, 19–28 (1969).
Matteson, D. S. Methanetetraboronic and methanetriboronic esters as synthetic intermediates. Synthesis 1975, 147–158 (1975).
Batsanov, A. S. et al. Fully borylated methane and ethane by ruthenium-mediated cleavage and coupling of CO. Angew. Chem. Int. Ed. 55, 4707–4710 (2016).
Liu, X.-W., Echavarren, J., Zarate, C. & Martin, R. Ni-catalyzed borylation of aryl fluorides via C–F cleavage. J. Am. Chem. Soc. 137, 12470–12473 (2015).
Niwa, T., Ochiai, H., Watanabe, Y. & Hosoya, T. Ni/Cu-catalyzed defluoroborylation of fluoroarenes for diverse C–F bond functionalizations. J. Am. Chem. Soc. 137, 14313–14318 (2015).
Zhou, J. et al. Preparing (multi)fluoroarenes as building blocks for synthesis: nickel-catalyzed borylation of polyfluoroarenes via C–F bond cleavage. J. Am. Chem. Soc. 138, 5250–5253 (2016).
Zhang, J., Dai, W., Liu, Q. & Cao, S. Cu-catalyzed stereoselective borylation of gem-difluoroalkenes with B2pin2. Org. Lett. 19, 3283–3286 (2017).
Sakaguchi, H. et al. Copper-catalyzed regioselective monodefluoroborylation of polyfluoroalkenes en route to diverse fluoroalkenes. J. Am. Chem. Soc. 139, 12855–12862 (2017).
Tan, D.-H. et al. Copper-catalyzed stereoselective defluorinative borylation and silylation of gem-difluoroalkenes. Adv. Synth. Catal. 360, 1032–1037 (2010).
Takachi, M., Kita, Y., Tobisu, M., Fukumoto, Y. & Chatani, N. Nickel-catalyzed cyclization of difluoro-substituted 1,6-enynes with organozinc reagents through the stereoselective activation of C–F bonds: synthesis of bicyclo[3.2.0]heptene derivatives. Angew. Chem. Int. Ed. 49, 8717–8720 (2010).
Gao, B., Zhao, Y. & Hu, J. AgF-mediated fluorinative cross-coupling of two olefins: facile access to α-CF3 alkenes and β-CF3 ketones. Angew. Chem. Int. Ed. 54, 638–642 (2015).
Tian, P., Feng, C. & Loh, T.-P. Rhodium-catalysed C(sp 2)–C(sp 2) bond formation via C–H/C–F activation. Nat. Commun. 6, 7472–7478 (2015).
Xie, J., Yu, J., Rudolph, M., Rominger, F. & Hashmi, A. S. K. Mono-fluoroalkenylation of dimethylamino compounds through radical–radical cross-coupling. Angew. Chem. Int. Ed. 55, 9416–9421 (2016).
Tian, P. et al. F− nucleophilic-addition-induced allylic alkylation. J. Am. Chem. Soc. 138, 15869–15872 (2016).
Thornbury, R. T. & Toste, F. D. Palladium-catalyzed defluorinative coupling of 1-aryl-2,2-difluoroalkenes and boronic acids: stereoselective synthesis of monofluorostilbenes. Angew. Chem. Int. Ed. 55, 11629–11632 (2016).
Tang, H.-J., Lin, L.-Z., Feng, C. & Loh, T.-P. Palladium-catalyzed fluoroarylation of gem-difluoroalkenes. Angew. Chem. Int. Ed. 56, 9872–9876 (2017).
Wu, J.-Q. et al. Experimental and theoretical studies on rhodium-catalyzed coupling of benzamides with 2,2-difluorovinyl tosylate: diverse synthesis of fluorinated heterocycles. J. Am. Chem. Soc. 139, 3537–3545 (2017).
Lu, X. et al. Nickel-catalyzed defluorinative reductive cross-coupling of gem-difluoroalkenes with unactivated secondary and tertiary alkyl halides. J. Am. Chem. Soc. 139, 12632–12637 (2017).
Liu, Y., Zhou, Y., Zhao, Y. & Qu, J. Synthesis of gem-difluoroallylboronates via FeCl2‑catalyzed boration/β-fluorine elimination of trifluoromethyl alkenes. Org. Lett. 19, 946–949 (2017).
Hu, J., Han, X., Yuan, Y. & Shi, Z. Stereoselective synthesis of Z fluoroalkenes through copper-catalyzed hydrodefluorination of gem-difluoroalkenes with water. Angew. Chem. Int. Ed. 56, 13342–13346 (2017).
Kojima, R., Kubota, K. & Ito, H. Stereodivergent hydrodefluorination of gem-difluoroalkenes: selective synthesis of (Z)- and (E)-monofluoroalkenes. Chem. Commun. 53, 10688–10691 (2017).
Hong, K., Liu, X. & Morken, J. P. Simple access to elusive α-boryl carbanions and their alkylation: an umpolung construction for organic synthesis. J. Am. Chem. Soc. 136, 10581–10584 (2014).
Acknowledgements
We thank A. Lei at Wuhan University for assistance with the operando infrared experiments. This work was supported by the Funding Programs for the ‘1000 Youth Talents Plan’, National Natural Science Foundation of China (grant 2167020084), ‘Jiangsu Specially-Appointed Professor Plan’ and ‘Innovation and Entrepreneurship Talents Plan’ of Jiangsu Province.
Author information
Authors and Affiliations
Contributions
Z.S. conceived the study, supervised the project and wrote the paper. J.H performed the experiments and mechanism study, and analysed the data. Y.Z. performed the crystallographic studies.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information
Supplementary Methods, Supplementary References, Supplementary Figures 1–108
Compound 5f
Crystallographic data for compound 5f
Compound 6f
Crystallographic data for compound 6f
Rights and permissions
About this article
Cite this article
Hu, J., Zhao, Y. & Shi, Z. Highly tunable multi-borylation of gem-difluoroalkenes via copper catalysis. Nat Catal 1, 860–869 (2018). https://doi.org/10.1038/s41929-018-0147-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-018-0147-9
This article is cited by
-
Stereodefined polymetalloid alkenes synthesis via stereoselective boron-masking of polyborylated alkenes
Nature Communications (2023)
-
Regio- and enantioselective nucleophilic addition to gem-difluoroallenes
Nature Synthesis (2022)
-
Photo-induced trifunctionalization of bromostyrenes via remote radical migration reactions of tetracoordinate boron species
Nature Communications (2022)
-
Diverse reactivity of the gem-difluorovinyl iodonium salt for direct incorporation of the difluoroethylene group into N- and O-nucleophiles
Communications Chemistry (2022)
-
Cobalt-catalyzed deoxygenative triborylation of allylic ethers to access 1,1,3-triborylalkanes
Nature Communications (2020)