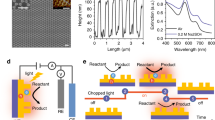
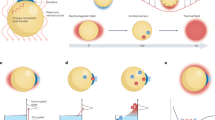
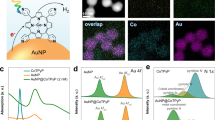
Abstract
The demonstrations of visible-light-driven chemical transformations on plasmonic metal nanostructures have led to the emergence of a new field in heterogeneous catalysis known as plasmonic catalysis. The excitement surrounding plasmonic catalysis stems from the ability to use the excitation of energetic charge carriers (as opposed to heat) to drive surface chemistry. This offers the opportunity to potentially discover new, more selective reaction pathways that cannot be accessed in temperature-driven catalysis. In this Review, we provide a fundamental overview of plasmonic catalysis with emphasis on recent advancements in the field. It is our objective to stress the importance of the underlying physical mechanisms at play in plasmonic catalysis and discuss possibilities and limitations in the field guided by these physical insights.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ertl, G. Reactions at surfaces: from atoms to complexity (Nobel Lecture). Angew. Chem. Int. Ed. 47, 3524–3535 (2008).
Hinrichsen, O., Rosowski, F., Hornung, A., Muhler, M. & Ertl, G. The kinetics of ammonia synthesis over Ru-based catalysts: 1. The dissociative chemisorption and associative desorption of N2. J. Catal. 165, 33–44 (1997).
Haryanto, A., Fernando, S., Murali, N. & Adhikari, S. Current status of hydrogen production techniques by steam reforming of ethanol: a review. Energy Fuels 19, 2098–2106 (2005).
Nikolla, E., Holewinski, A., Schwank, J. & Linic, S. Controlling carbon surface chemistry by alloying: carbon tolerant reforming catalyst. J. Am. Chem. Soc. 128, 11354–11355 (2006).
Studt, F. et al. Identification of non-precious metal alloy catalysts for selective hydrogenation of acetylene. Science 320, 1320–1322 (2008).
Kliewer, C. J., Bieri, M. & Somorjai, G. A. Hydrogenation of the α,β-unsaturated aldehydes acrolein, crotonaldehyde, and prenal over Pt single crystals: a kinetic and sum-frequency generation vibrational spectroscopy study. J. Am. Chem. Soc. 131, 9958–9966 (2009).
Saavedra, J. et al. Controlling activity and selectivity using water in the Au-catalysed preferential oxidation of CO in H2. Nat. Chem. 8, 584–589 (2016).
Dellamorte, J. C., Lauterbach, J. & Barteau, M. A. Palladium–silver bimetallic catalysts with improved activity and selectivity for ethylene epoxidation. Appl. Catal. Gen. 391, 281–288 (2011).
Grabow, L. C., Gokhale, A. A., Evans, S. T., Dumesic, J. A. & Mavrikakis, M. Mechanism of the water gas shift reaction on Pt: first principles, experiments, and microkinetic modeling. J. Phys. Chem. C 112, 4608–4617 (2008).
Stamenkovic, V. R. et al. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science 315, 493–497 (2007).
Cleve, T. V., Moniri, S., Belok, G., More, K. L. & Linic, S. Nanoscale engineering of efficient oxygen reduction electrocatalysts by tailoring the local chemical environment of Pt surface sites. ACS Catal. 7, 17–24 (2017).
Hu, B. et al. Selective propane dehydrogenation with single-site Coii on SiO2 by a non-redox mechanism. J. Catal. 322, 24–37 (2015).
Christopher, P. & Linic, S. Shape- and size-specific chemistry of Ag nanostructures in catalytic ethylene epoxidation. ChemCatChem 2, 78–83 (2010).
Holewinski, A., Xin, H., Nikolla, E. & Linic, S. Identifying optimal active sites for heterogeneous catalysis by metal alloys based on molecular descriptors and electronic structure engineering. Curr. Opin. Chem. Eng. 2, 312–319 (2013).
Schweitzer, N., Xin, H., Nikolla, E., Miller, J. T. & Linic, S. Establishing relationships between the geometric structure and chemical reactivity of alloy catalysts based on their measured electronic structure. Top. Catal. 53, 348–356 (2010).
Lindstrom, C. D. & Zhu, X.-Y. Photoinduced electron transfer at molecule–metal interfaces. Chem. Rev. 106, 4281–4300 (2006). Review paper summarizing mechanisms of electron transfer at molecule–metal interfaces with emphasis on the role of chemical bonding at the interface.
Frischkorn, C. & Wolf, M. Femtochemistry at metal surfaces: nonadiabatic reaction dynamics. Chem. Rev. 106, 4207–4233 (2006).
White, J. M. Using photons and electrons to drive surface chemical reactions. J. Mol. Catal. Chem. 131, 71–90 (1998).
Busch, D. G. & Ho, W. Direct observation of the crossover from single to multiple excitations in femtosecond surface photochemistry. Phys. Rev. Lett. 77, 1338–1341 (1996).
Linic, S., Christopher, P., Xin, H. & Marimuthu, A. Catalytic and photocatalytic transformations on metal nanoparticles with targeted geometric and plasmonic properties. Acc. Chem. Res. 46, 1890–1899 (2013).
Buntin, S., Richter, L., Cavanagh, R. & King, D. Optically driven surface reactions: evidence for the role of hot electrons. Phys. Rev. Lett. 61, 1321–1324 (1988).
Deliwala, S. et al. Surface femtochemistry of O2 and CO on Pt(111). Chem. Phys. Lett. 242, 617–622 (1995).
Bonn, M. et al. Phonon- versus electron-mediated desorption and oxidation of CO on Ru(0001). Science 285, 1042–1045 (1999). Demonstration of unique chemical reaction outcomes for electron-mediated processes versus phonon-mediated processes on bulk metals under laser excitation.
Hertel, T., Knoesel, E., Wolf, M. & Ertl, G. Ultrafast electron dynamics at Cu(111): response of an electron gas to optical excitation. Phys. Rev. Lett. 76, 535–538 (1996).
Christopher, P., Xin, H. & Linic, S. Visible-light-enhanced catalytic oxidation reactions on plasmonic silver nanostructures. Nat. Chem. 3, 467–472 (2011). Demonstration of visible-light-enhanced reactions on plasmonic Ag nanostructures with systematic experiments uncovering the role of hot charge carriers in activating chemical bonds .
Mukherjee, S. et al. Hot electrons do the impossible: plasmon-induced dissociation of H2 on Au. Nano Lett. 13, 240–247 (2013).
Marimuthu, A., Zhang, J. & Linic, S. Tuning selectivity in propylene epoxidation by plasmon mediated photo-switching of Cu oxidation state. Science 339, 1590–1593 (2013). A unique case in which plasmon excitation causes a change in the oxidation state of a metal catalyst under operating conditions resulting in a significant improvement of product selectivity.
Linic, S., Aslam, U., Boerigter, C. & Morabito, M. Photochemical transformations on plasmonic metal nanoparticles. Nat. Mater. 14, 567–576 (2015).
Kale, M. J., Avanesian, T. & Christopher, P. Direct photocatalysis by plasmonic nanostructures. ACS Catal. 4, 116–128 (2014).
Zhang, Y. et al. Surface-plasmon-driven hot electron photochemistry. Chem. Rev. 118, 2927–2954 (2018).
Zhang, X., Chen, Y. L., Liu, R.-S. & Tsai, D. P. Plasmonic photocatalysis. Rep. Prog. Phys. 76, 046401 (2013).
Maier, S. A. Plasmonics: Fundamentals and Applications (Springer Science and Business Media, Bath, 2007).
Kelly, K. L., Coronado, E., Zhao, L. L. & Schatz, G. C. The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J. Phys. Chem. B 107, 668–677 (2002).
Link, S. & El-Sayed, M. A. Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods. J. Phys. Chem. B 103, 8410–8426 (1999).
El-Sayed, M. A. Some interesting properties of metals confined in time and nanometer space of different shapes. Acc. Chem. Res. 34, 257–264 (2001).
Langhammer, C., Kasemo, B. & Zorić, I. Absorption and scattering of light by Pt, Pd, Ag, and Au nanodisks: absolute cross sections and branching ratios. J. Chem. Phys. 126, 194702 (2007).
Kreibig, U. & Vollmer, M. Optical Properties of Metal Clusters (Springer Science and Business Media, Heidelberg, 2013). Provides a comprehensive introduction to plasmonic excitation in metal nanoparticles.
Stiles, P. L., Dieringer, J. A., Shah, N. C. & Duyne, R. P. V. Surface-enhanced raman spectroscopy. Annu. Rev. Anal. Chem. 1, 601–626 (2008).
Khurgin, J. B. How to deal with the loss in plasmonics and metamaterials. Nat. Nanotech. 10, 2–6 (2015).
Halas, N. J., Lal, S., Chang, W.-S., Link, S. & Nordlander, P. Plasmons in strongly coupled metallic nanostructures. Chem. Rev. 111, 3913–3961 (2011).
Hao, E. & Schatz, G. C. Electromagnetic fields around silver nanoparticles and dimers. J. Chem. Phys. 120, 357–366 (2004).
Ingram, D. B. & Linic, S. Water splitting on composite plasmonic-metal/semiconductor photoelectrodes: evidence for selective plasmon-induced formation of charge carriers near the semiconductor surface. J. Am. Chem. Soc. 133, 5202–5205 (2011).
Linic, S., Christopher, P. & Ingram, D. B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 10, 911–921 (2011).
Kambhampati, P., Child, C. M., Foster, M. C. & Campion, A. On the chemical mechanism of surface enhanced Raman scattering: experiment and theory. J. Chem. Phys. 108, 5013–5026 (1998).
Jain, P. K., Huang, X., El-Sayed, I. H. & El-Sayed, M. A. Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 41, 1578–1586 (2008).
Dolinnyi, A. I. Nanometric rulers based on plasmon coupling in pairs of gold nanoparticles. J. Phys. Chem. C 119, 4990–5001 (2015).
Nasir, M. E., Dickson, W., Wurtz, G. A., Wardley, W. P. & Zayats, A. V. Hydrogen detected by the naked eye: optical hydrogen gas sensors based on core/shell plasmonic nanorod metamaterials. Adv. Mater. 26, 3532–3537 (2014).
Carpin, L. B. et al. Immunoconjugated gold nanoshell-mediated photothermal ablation of trastuzumab-resistant breast cancer cells. Breast Cancer Res. Treat. 125, 27–34 (2010).
El-Sayed, I. H., Huang, X. & El-Sayed, M. A. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 239, 129–135 (2006).
Neumann, O. et al. Nanoparticle-mediated, light-induced phase separations. Nano Lett. 15, 7880–7885 (2015).
Brus, L. Noble metal nanocrystals: plasmon electron transfer photochemistry and single-molecule Raman spectroscopy. Acc. Chem. Res. 41, 1742–1749 (2008). Article highlighting strong light–matter interactions among plasmonic nanostructures and molecules.
Moskovits, M. Surface-enhanced spectroscopy. Rev. Mod. Phys. 57, 783–826 (1985).
Moskovits, M. Surface-enhanced Raman spectroscopy: a brief retrospective. J. Raman Spectrosc. 36, 485–496 (2005).
Kneipp, K. et al. Near-infrared surface-enhanced Raman scattering can detect single molecules and observe ‘hot’ vibrational transitions. J. Raman Spectrosc. 29, 743–747 (1998).
Jiang, Bosnick, K., Maillard, M. & Brus, L. Single molecule Raman spectroscopy at the junctions of large Ag nanocrystals. J. Phys. Chem. B 107, 9964–9972 (2003).
Kneipp, K. et al. Single molecule detection using surface-enhanced Raman scattering (SERS). Phys. Rev. Lett. 78, 1667–1670 (1997).
Evanoff, D. D. & Chumanov, G. Size-controlled synthesis of nanoparticles. 2. Measurement of extinction, scattering, and absorption cross sections. J. Phys. Chem. B 108, 13957–13962 (2004).
Jain, P. K., Lee, K. S., El-Sayed, I. H. & El-Sayed, M. A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine. J. Phys. Chem. B 110, 7238–7248 (2006).
Bosbach, J., Hendrich, C., Stietz, F., Vartanyan, T. & Träger, F. Ultrafast dephasing of surface plasmon excitation in silver nanoparticles: influence of particle size, shape, and chemical surrounding. Phys. Rev. Lett. 89, 257404 (2002).
Manjavacas, A., Liu, J. G., Kulkarni, V. & Nordlander, P. Plasmon-induced hot carriers in metallic nanoparticles. ACS Nano 8, 7630–7638 (2014).
Atwater, H. A. & Polman, A. Plasmonics for improved photovoltaic devices. Nat. Mater. 9, 205–213 (2010).
Brown, A. M., Sundararaman, R., Narang, P., Goddard, W. A. & Atwater, H. A. Nonradiative plasmon decay and hot carrier dynamics: effects of phonons, surfaces, and geometry. ACS Nano 10, 957–966 (2016). First-principles calculations of plasmon decay pathways in plasmonic nanostructures with analysis relating the decay pathways to the physical properties of the metals.
Bernardi, M., Mustafa, J., Neaton, J. B. & Louie, S. G. Theory and computation of hot carriers generated by surface plasmon polaritons in noble metals. Nat. Commun. 6, 7044 (2015).
Sundararaman, R., Narang, P., Jermyn, A. S., Goddard Iii, W. A. & Atwater, H. A. Theoretical predictions for hot-carrier generation from surface plasmon decay. Nat. Commun. 5, 5788 (2014).
Brongersma, M. L., Halas, N. J. & Nordlander, P. Plasmon-induced hot carrier science and technology. Nat. Nanotech. 10, 25–34 (2015).
Knoesel, E., Hotzel, A. & Wolf, M. Ultrafast dynamics of hot electrons and holes in copper: excitation, energy relaxation, and transport effects. Phys. Rev. B 57, 12812–12824 (1998).
Christopher, P., Xin, H., Marimuthu, A. & Linic, S. Singular characteristics and unique chemical bond activation mechanisms of photocatalytic reactions on plasmonic nanostructures. Nat. Mater. 11, 1044–1050 (2012). Combined experimental and theoretical study uncovering the role of hot carriers in plasmon-driven chemical reactions.
Mukherjee, S. et al. Hot-electron-induced dissociation of H2 on gold nanoparticles supported on SiO2. J. Am. Chem. Soc. 136, 64–67 (2014).
Landry, M. J., Gellé, A., Meng, B. Y., Barrett, C. J. & Moores, A. Surface-plasmon-mediated hydrogenation of carbonyls catalyzed by silver nanocubes under visible light. ACS Catal. 7, 6128–6133 (2017).
Zhu, H., Ke, X., Yang, X., Sarina, S. & Liu, H. Reduction of nitroaromatic compounds on supported gold nanoparticles by visible and ultraviolet light. Angew. Chem. 122, 9851–9855 (2010).
Kim, Y., Wilson, A. J. & Jain, P. K. The nature of plasmonically assisted hot-electron transfer in a donor–bridge–acceptor complex. ACS Catal. 7, 4360–4365 (2017).
Huang, Y.-F. et al. Activation of oxygen on gold and silver nanoparticles assisted by surface plasmon resonances. Angew. Chem. Int. Ed. 53, 2353–2357 (2014).
Kim, Y., Dumett Torres, D. & Jain, P. K. Activation energies of plasmonic catalysts. Nano Lett. 16, 3399–3407 (2016).
Upadhye, A. A. et al. Plasmon-enhanced reverse water gas shift reaction over oxide supported Au catalysts. Catal. Sci. Technol. 5, 2590–2601 (2015).
Kazuma, E., Jung, J., Ueba, H., Trenary, M. & Kim, Y. Direct pathway to molecular photodissociation on metal surfaces using visible light. J. Am. Chem. Soc. 139, 3115–3121 (2017).
Zhang, J. et al. Ag@Au concave cuboctahedra: a unique probe for monitoring Au-catalyzed reduction and oxidation reactions by surface-enhanced Raman spectroscopy. ACS Nano 10, 2607–2616 (2016).
Xie, W. & Schlücker, S. Hot electron-induced reduction of small molecules on photorecycling metal surfaces. Nat. Commun. 6, 7570 (2015).
Zhang, X. et al. Product selectivity in plasmonic photocatalysis for carbon dioxide hydrogenation. Nat. Commun. 8, 14542 (2017).
Ageev, V. N. Desorption induced by electronic transitions. Prog. Surf. Sci. 47, 55–203 (1994).
Misewich, J. A., Heinz, T. F. & Newns, D. M. Desorption induced by multiple electronic transitions. Phys. Rev. Lett. 68, 3737–3740 (1992).
Shirhatti, P. R. et al. Observation of the adsorption and desorption of vibrationally excited molecules on a metal surface. Nat. Chem. 10, 592–598 (2018).
Kale, M. J., Avanesian, T., Xin, H., Yan, J. & Christopher, P. Controlling catalytic selectivity on metal nanoparticles by direct photoexcitation of adsorbate–metal bonds. Nano Lett. 14, 5405–5412 (2014).
Boerigter, C., Campana, R., Morabito, M. & Linic, S. Evidence and implications of direct charge excitation as the dominant mechanism in plasmon-mediated photocatalysis. Nat. Commun. 7, 10545 (2016).
Palmer, R. E. & Rous, P. J. Resonances in electron scattering by molecules on surfaces. Rev. Mod. Phys. 64, 383–440 (1992).
Yan, J., Jacobsen, K. W. & Thygesen, K. S. First-principles study of surface plasmons on Ag(111) and H/Ag(111). Phys. Rev. B 84, 235430 (2011).
Boerigter, C., Aslam, U. & Linic, S. Mechanism of charge transfer from plasmonic nanostructures to chemically attached materials. ACS Nano 10, 6108–6115 (2016). Mechanistic study and analysis of energy transfer from plasmonic metals to molecules demonstrating the dominant role of the direct energy transfer mechanism.
Browne, W. R. & McGarvey, J. J. The Raman effect and its application to electronic spectroscopies in metal-centered species: techniques and investigations in ground and excited states. Coord. Chem. Rev. 251, 454–473 (2007).
Wu, K., Chen, J., McBride, J. R. & Lian, T. Efficient hot-electron transfer by a plasmon-induced interfacial charge-transfer transition. Science 349, 632–635 (2015).
Tan, S. et al. Plasmonic coupling at a metal/semiconductor interface. Nat. Photon. 11, 806–812 (2017).
Kazuma, E., Jung, J., Ueba, H., Trenary, M. & Kim, Y. Real-space and real-time observation of a plasmon-induced chemical reaction of a single molecule. Science 360, 521–526 (2018).
DuChene, J. S., Tagliabue, G., Welch, A. J., Cheng, W.-H. & Atwater, H. A. Hot hole collection and photoelectrochemical CO2 reduction with plasmonic Au/p-GaN photocathodes. Nano Lett. 18, 2545–2550 (2018).
Bauer, C., Abid, J.-P., Fermin, D. & Girault, H. H. Ultrafast chemical interface scattering as an additional decay channel for nascent nonthermal electrons in small metal nanoparticles. J. Chem. Phys. 120, 9302–9315 (2004).
Hendrich, C. et al. Chemical interface damping of surface plasmon excitation in metal nanoparticles: a study by persistent spectral hole burning. Appl. Phys. B 76, 869–875 (2003).
Foerster, B. et al. Chemical interface damping depends on electrons reaching the surface. ACS Nano 11, 2886–2893 (2017).
Linnert, T., Mulvaney, P. & Henglein, A. Surface chemistry of colloidal silver: surface plasmon damping by chemisorbed iodide, hydrosulfide (SH−), and phenylthiolate. J. Phys. Chem. 97, 679–682 (1993).
Aslam, U., Chavez, S. & Linic, S. Controlling energy flow in multimetallic nanostructures for plasmonic catalysis. Nat. Nanotech. 12, 1000–1005 (2017). Systematic study illuminating the mechanism of energy transfer from plasmonic metals to catalytic metals.
Swearer, D. F. et al. Heterometallic antenna–reactor complexes for photocatalysis. Proc. Natl Acad. Sci. USA 113, 8916–8920 (2016).
Zhang, C. et al. Al–Pd nanodisk heterodimers as antenna–reactor photocatalysts. Nano Lett. 16, 6677–6682 (2016).
Mukherjee, J. & Linic, S. First-principles investigations of electrochemical oxidation of hydrogen at solid oxide fuel cell operating conditions. J. Electrochem. Soc. 154, B919–B924 (2007).
Ingram, D. B. & Linic, S. First-principles analysis of the activity of transition and noble metals in the direct utilization of hydrocarbon fuels at solid oxide fuel cell operating conditions. J. Electrochem. Soc. 156, B1457–B1465 (2009).
Acknowledgements
The work presented in this document was supported by the National Science Foundation (NSF) (CBET-1702471 and CHE- 1800197) (optical analysis) and Office of Basic Energy Science, Division of Chemical Sciences (FG-02-05ER15686) (materials synthesis). Secondary support for the development of analytical tools used to analyse the reaction kinetics was provided by the NSF (CBET-1436056). S.L. also acknowledges the partial support of the Technische Universität München – Institute for Advanced Study, funded by the German Excellence Initiative and the European Union Seventh Framework Programme under grant agreement no. 291763.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Additional information
Reprints and permissions information is available at www.nature.com/reprints.
Correspondence should be addressed to S.L.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aslam, U., Rao, V.G., Chavez, S. et al. Catalytic conversion of solar to chemical energy on plasmonic metal nanostructures. Nat Catal 1, 656–665 (2018). https://doi.org/10.1038/s41929-018-0138-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-018-0138-x
This article is cited by
-
Pt-doped Ru nanoparticles loaded on ‘black gold’ plasmonic nanoreactors as air stable reduction catalysts
Nature Communications (2024)
-
Atomic reconstruction for realizing stable solar-driven reversible hydrogen storage of magnesium hydride
Nature Communications (2024)
-
Exploiting hot electrons from a plasmon nanohybrid system for the photoelectroreduction of CO2
Communications Chemistry (2024)
-
Hydrogen evolution with hot electrons on a plasmonic-molecular catalyst hybrid system
Nature Communications (2024)
-
Plasmonic bimetallic two-dimensional supercrystals for H2 generation
Nature Catalysis (2023)