Abstract
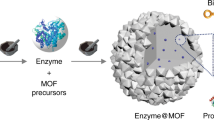
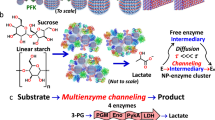
Biocatalytic transformations in cells, such as enzyme cascades, involve complex networks proceeding in spatially confined microenvironments. Here, inspired by nature, we demonstrate effective biocatalytic cascades by the encapsulation of two or three enzymes, or enzyme/cofactor components, in zeolitic imidazolate framework-8 metal–organic framework nanoparticles (ZIF8-NMOFs) that act as nanoreactors. The integration of the two-enzyme system (glucose oxidase and horseradish peroxidase) or three-enzyme system (β-galactosidase, glucose oxidase and horseradish peroxidase) in the NMOFs leads to 7.5-fold and 5.3-fold enhancements in the activity of the catalytic cascades, respectively, compared with the bulk mixture of the catalysts in solution. In addition, the encapsulation of alcohol dehydrogenase, NAD+–polymer and lactate dehydrogenase in the NMOFs yields a coupled biocatalytic cascade involving coupled NAD+-dependent enzymes, leading to the catalytic reduction of pyruvic acid to lactic acid by ethanol.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Good, M. C., Zalatan, J. G. & Lim, W. A. Scaffold proteins: hubs for controlling the flow of cellular information. Science 332, 680–686 (2011).
Cooper, G. M. The Cell: A Molecular Approach (Sinauer Associates, Sunderland, 2000).
Barabási, A. L. & Oltvai, Z. N. Network biology: understanding the cell’s functional organization. Nat. Rev. Genet. 5, 101–113 (2004).
Yeger-Lotem, E. et al. Network motifs in integrated cellular networks of transcription–regulation and protein–protein interaction. Proc. Natl Acad. Sci. USA 101, 5934–5939 (2004).
Davidson, E. & Levin, M. Gene regulatory networks. Proc. Natl Acad. Sci. USA 102, 4935–4942 (2005).
Quin, M. B., Wallin, K. K., Zhang, G. & Schmidt-Dannert, C. Spatial organization of multi-enzyme biocatalytic cascades. Org. Biomol. Chem. 15, 4260–4271 (2017).
Rabe, K. S., Müller, J., Skoupi, M. & Niemeyer, C. M. Cascades in compartments: en route to machine-assisted biotechnology. Angew. Chem. Int. Ed. 56, 13574–13589 (2017).
Gröger, H. & Hummel, W. Combining the ‘two worlds’ of chemocatalysis and biocatalysis towards multi-step one-pot processes in aqueous media. Curr. Opin. Chem. Biol. 19, 171–179 (2014).
Li, J. et al. Synthesis of many different types of organic small molecules using one automated process. Science 347, 1221–1226 (2015).
Agapakis, C. M., Boyle, P. M. & Silver, P. A. Natural strategies for the spatial optimization of metabolism in synthetic biology. Nat. Chem. Biol. 8, 527–535 (2012).
Both, P. et al. Whole-cell biocatalysts for stereoselective C–H amination reactions. Angew. Chem. Int. Ed. 55, 1511–1513 (2016).
Bayer, E. A., Belaich, J. P., Shoham, Y. & Lamed, R. The cellulosomes: multienzyme machines for degradation of plant cell wall polysaccharides. Annu. Rev. Microbiol. 58, 521–554 (2004).
Laursen, T. et al. Characterization of a dynamic metabolon producing the defense compound dhurrin in sorghum. Science 354, 890–893 (2016).
Schoffelen, S. & van Hest, J. C. Chemical approaches for the construction of multi-enzyme reaction systems. Curr. Opin. Struct. Biol. 23, 613–621 (2013).
Vance, S. et al. Sticky swinging arm dynamics: studies of an acyl carrier protein domain from the mycolactone polyketide synthase. Biochem. J. 473, 1097–1110 (2016).
Delebecque, C. J., Lindner, A. B., Silver, P. A. & Aldaye, F. A. Organization of intracellular reactions with rationally designed RNA assemblies. Science 333, 470–474 (2011).
Wilner, O. I. et al. Enzyme cascades activated on topologically programmed DNA scaffolds. Nat. Nanotech. 4, 249–254 (2009).
Wang, Z. G., Wilner, O. I. & Willner, I. Self-assembly of aptamer-circular DNA nanostructures for controlled biocatalysis. Nano Lett. 9, 4098–4102 (2009).
Fu, J., Liu, M., Liu, Y., Woodbury, N. W. & Yan, H. Interenzyme substrate diffusion for an enzyme cascade organized on spatially addressable DNA nanostructures. J. Am. Chem. Soc. 134, 5516–5519 (2012).
Timm, C. & Niemeyer, C. M. Assembly and purification of enzyme-functionalized DNA origami structures. Angew. Chem. Int. Ed. 54, 6745–6750 (2015).
Liu, M. et al. A DNA tweezer-actuated enzyme nanoreactor. Nat. Commun. 4, 2127 (2013).
Aleman-Garcia, M. A., Orbach, R. & Willner, I. Ion-responsive hemin-G-quadruplexes for switchable DNAzyme and enzyme functions. Chem. Eur. J. 20, 5619–5624 (2014).
Wang, Z. & Cohen, S. M. Postsynthetic modification of metal–organic frameworks. Chem. Soc. Rev. 38, 1315–1329 (2009).
Furukawa, H., Cordova, K. E., O’Keeffe, M. & Yaghi, O. M. The chemistry and applications of metal–organic frameworks. Science 341, 1230444 (2013).
Cui, Y. et al. Metal–organic frameworks as platforms for functional materials. Acc. Chem. Res. 49, 483–493 (2016).
Stavila, V. et al. MOF-based catalysts for selective hydrogenolysis of carbon–oxygen ether bonds. ACS Catal. 6, 55–59 (2015).
Yoon, M., Srirambalaji, R. & Kim, K. Homochiral metal–organic frameworks for asymmetric heterogeneous catalysis. Chem. Rev. 112, 1196–1231 (2012).
Liu, J. et al. Applications of metal–organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 43, 6011–6061 (2014).
Liu, D., Huxford, R. C. & Lin, W. Phosphorescent nanoscale coordination polymers as contrast agents for optical imaging. Angew. Chem. Int. Ed. 50, 3696–3700 (2011).
Horcajada, P. et al. Metal–organic frameworks in biomedicine. Chem. Rev. 112, 1232–1268 (2012).
Chen, W. H. et al. ATP-responsive aptamer-based metal–organic framework nanoparticles (NMOFs) for the controlled release of loads and drugs. Adv. Funct. Mater. 27, 1702102 (2017).
Chen, W. H. et al. Stimuli-responsive nucleic acid-functionalized metal–organic framework nanoparticles using pH- and metal-ion-dependent DNAzymes as locks. Chem. Sci. 8, 5769–5780 (2017).
Chen, W. H. et al. Stimuli-responsive nucleic acid-based polyacrylamide hydrogel-coated metal–organic framework nanoparticles for controlled drug release. Adv. Funct. Mater. 28, 1705137 (2018).
Kreno, L. E. et al. Metal–organic framework materials as chemical sensors. Chem. Rev. 112, 1105–1125 (2012).
Wu, L. L. et al. A metal–organic framework/DNA hybrid system as a novel fluorescent biosensor for mercury(II) ion detection. Chem. Eur. J. 22, 477–480 (2016).
Della Rocca, J., Liu, D. & Lin, W. Nanoscale metal–organic frameworks for biomedical imaging and drug delivery. Acc. Chem. Res. 44, 957–968 (2011).
Taylor, K. M., Jin, A. & Lin, W. Surfactant-assisted synthesis of nanoscale gadolinium metal–organic frameworks for potential multimodal imaging. Angew. Chem. Int. Ed. 47, 7722–7725 (2008).
Li, S. L. & Xu, Q. Metal–organic frameworks as platforms for clean energy. Energy Environ. Sci. 6, 1656–1683 (2013).
Hurd, J. A. et al. Anhydrous proton conduction at 150 °C in a crystalline metal–organic framework. Nat. Chem. 1, 705–710 (2009).
Lian, X. et al. Enzyme-MOF (metal–organic framework) composites. Chem. Soc. Rev. 46, 3386–3401 (2017).
Lian, X., Chen, Y. P., Liu, T. F. & Zhou, H. C. Coupling two enzymes into a tandem nanoreactor utilizing a hierarchically structured MOF. Chem. Sci. 7, 6969–6973 (2016).
Ge, J., Lei, J. & Zare, R. N. Protein–inorganic hybrid nanoflowers. Nat. Nanotechnol. 7, 428–432 (2012).
Doonan, C., Riccò, R., Liang, K., Bradshaw, D. & Falcaro, P. Metal–organic frameworks at the biointerface: synthetic strategies and applications. Acc. Chem. Res. 50, 1423–1432 (2017).
Zhuang, J., Young, A. P. & Tsung, C. K. Integration of biomolecules with metal–organic frameworks. Small 13, 1700880 (2017).
Wu, X., Ge, J., Yang, C., Hou, M. & Liu, Z. Facile synthesis of multiple enzyme-containing metal–organic frameworks in a biomolecule-friendly environment. Chem. Commun. 51, 13408–13411 (2015).
Wang, Q., Zhang, X., Huang, L., Zhang, Z. & Dong, S. GOx@ZIF-8(NiPd) nanoflower: an artificial enzyme system for tandem catalysis. Angew. Chem. Int. Ed. 56, 16082–16085 (2017).
Liang, K. et al. Biomimetic mineralization of metal–organic frameworks as protective coatings for biomacromolecules. Nat. Commun. 6, 7240 (2015).
Karagiaridi, O. et al. Opening ZIF-8: a catalytically active zeolitic imidazolate framework of sodalite topology with unsubstituted linkers. J. Am. Chem. Soc. 134, 18790–18796 (2012).
Takahashi, H. et al. Immobilized enzymes in ordered mesoporous silica materials and improvement of their stability and catalytic activity in an organic solvent. Microporous Mesoporous Mater. 44, 755–762 (2001).
Lei, C., Shin, Y., Liu, J. & Ackerman, E. J. Entrapping enzyme in a functionalized nanoporous support. J. Am. Chem. Soc. 124, 11242–11243 (2002).
Kula, M. R. & Wandrey, C. Continuous enzymatic transformation in an enzyme-membrane reactor with simultaneous NADH regeneration. Methods Enzymol. 136, 9–21 (1987).
Wandrey, C., Liese, A. & Kihumbu, D. Industrial biocatalysis: past, present, and future. Org. Process Res. Dev. 4, 286–290 (2000).
Wichmann, R., Wandrey, C., Bückmann, A. F. & Kula, M. R. Continuous enzymatic transformation in an enzyme membrane reactor with simultaneous NAD(H) regeneration. Biotechnol. Bioeng. 67, 791–804 (2000).
Li, P. et al. Encapsulation of a nerve agent detoxifying enzyme by a mesoporous zirconium metal–organic framework engenders thermal and long-term stability. J. Am. Chem. Soc. 138, 8052–8055 (2016).
Li, P. et al. Nanosizing a metal–organic framework enzyme carrier for accelerating nerve agent hydrolysis. ACS Nano 10, 9174–9182 (2016).
Lyu, F., Zhang, Y., Zare, R. N., Ge, J. & Liu, Z. One-pot synthesis of protein-embedded metal–organic frameworks with enhanced biological activities. Nano Lett. 14, 5761–5765 (2014).
Liang, K. et al. Metal–organic framework coatings as cytoprotective exoskeletons for living cells. Adv. Mater. 28, 7910–7914 (2016).
Liang, K. et al. An enzyme-coated metal–organic framework shell for synthetically adaptive cell survival. Angew. Chem. Int. Ed. 129, 8630–8635 (2017).
Acknowledgements
This research was supported by the Israel Science Foundation. We thank M. Spira and S.-Y. Sung for assisting with the confocal microscopy experiments.
Author information
Authors and Affiliations
Contributions
W.-H.C. and M.V.-G. performed the experiments, analysed the results and participated in writing the paper. I.W. supervised the project. A.Z. and R.A.-R. helped to perform some of the analytic experiments related to this study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Discussion, Supplementary Figures 1–13, Supplementary Table 1, Supplementary References
Rights and permissions
About this article
Cite this article
Chen, WH., Vázquez-González, M., Zoabi, A. et al. Biocatalytic cascades driven by enzymes encapsulated in metal–organic framework nanoparticles. Nat Catal 1, 689–695 (2018). https://doi.org/10.1038/s41929-018-0117-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-018-0117-2
This article is cited by
-
Advances in cofactor immobilization for enhanced continuous-flow biocatalysis
Journal of Flow Chemistry (2024)
-
FeNi Prussian blue analogues on highly graphitized carbon nanosheets as efficient glucose sensors
Rare Metals (2024)
-
Pros and Cons in Various Immobilization Techniques and Carriers for Enzymes
Applied Biochemistry and Biotechnology (2024)
-
Recent progress in MOFs-based nanozymes for biosensing
Nano Research (2024)
-
Spatiotemporal control for integrated catalysis
Nature Reviews Methods Primers (2023)