Abstract
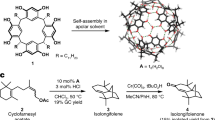
Terpenes constitute the largest class of natural products and serve as an important source for medicinal treatments. Despite constant progress in chemical synthesis, the construction of complex polycyclic sesqui- and diterpene scaffolds remains challenging. However, natural cyclase enzymes are able to form the whole variety of terpene structures from just a handful of linear precursors. Man-made catalysts able to mimic such natural enzymes are lacking. Here, we describe examples of sesquiterpene cyclizations inside an enzyme-mimicking supramolecular catalyst. This strategy allowed the formation of the tricyclic sesquiterpene isolongifolene in only four steps. The mechanism of the catalysed cyclization reaction was elucidated using 13C-labelling studies and density functional theory calculations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Maimone, T. J. & Baran, P. S. Modern synthetic efforts toward biologically active terpenes. Nat. Chem. Biol. 3, 396–407 (2007).
Urabe, D., Asaba, T. & Inoue, M. Convergent strategies in total syntheses of complex terpenoids. Chem. Rev. 115, 9207–9231 (2015).
Sobti, R. R. & Dev, S. Synthesis of (±)-isolongifolene. Tetrahedron Lett. 8, 2893–2895 (1967).
Sobti, R. R. & Dev, S. Studies in sesquiterpenes—XLIII: isolongifolene (part 4): synthesis. Tetrahedron 26, 649–655 (1970).
Volkmann, R. A., Andrews, G. C. & Johnson, W. S. Novel synthesis of longifolene. J. Am. Chem. Soc. 97, 4777–4779 (1975).
Arigoni, D. Stereochemical aspects of sesquiterpene biosynthesis. Pure Appl. Chem. 41, 219–245 (1975).
Berson, J. A. et al. Chemistry of methylnorbornyl cations. VI. The stereochemistry of vicinal hydride shift. Evidence for the nonclassical structure of 3-methyl-2-norbornyl cations. J. Am. Chem. Soc. 89, 2590–2600 (1967).
Yadav, J. S., Nayak, U. R. & Dev, S. Studies in sesquiterpenes—LV: isolongifolene(part 6): mechanism of rearrangement of longifolene to isolongifolene-I. Tetrahedron 36, 309–315 (1980).
Svensson, L. & Bendz, G. Essential oils from some liverworts. Phytochemistry 11, 1172–1173 (1972).
Pronin, S. V. & Shenvi, R. A. Synthesis of highly strained terpenes by non-stop tail-to-head polycyclization. Nat. Chem. 4, 915–920 (2012).
Yoder, R. A. & Johnston, J. N. A case study in biomimetic total synthesis: polyolefin carbocyclizations to terpenes and steroids. Chem. Rev. 105, 4730–4756 (2005).
Ohta, Y. & Hirose, Y. Electrophile induced cyclization of farnesol. Chem. Lett. 1, 263–266 (1972).
Andersen, N. H. & Syrdal, D. D. Chemical simulation of the biogenesis of cedrene. Tetrahedron Lett. 13, 2455–2458 (1972).
Polovinka, M. P. et al. Cyclization and rearrangements of farnesol and nerolidol stereoisomers in superacids. J. Org. Chem. 59, 1509–1517 (1994).
Gutsche, C. D., Maycock, J. R. & Chang, C. T. Acid-catalyzed cyclization of farnesol and nerolidol. Tetrahedron 24, 859–876 (1968).
Susumu, K., Mikio, T. & Teruaki, M. Biogenetic-like cyclization of farnesol and nerolidol to bisabolene by the use of 2-fluorobenzothiazolium salt. Chem. Lett. 6, 1169–1172 (1977).
Christianson, D. W. Structural biology and chemistry of the terpenoid cyclases. Chem. Rev. 106, 3412–3442 (2006).
Pluth, M. D., Bergman, R. G. & Raymond, K. N. Proton-mediated chemistry and catalysis in a self-assembled supramolecular host. Acc. Chem. Res. 42, 1650–1659 (2009).
Yoshizawa, M. & Fujita, M. Development of unique chemical phenomena within nanometer-sized, self-assembled coordination hosts. Bull. Chem. Soc. Jpn. 83, 609–618 (2010).
Ronson, T. K., Zarra, S., Black, S. P. & Nitschke, J. R. Metal–organic container molecules through subcomponent self-assembly. Chem. Commun. 49, 2476–2490 (2013).
Han, M., Engelhard, D. M. & Clever, G. H. Self-assembled coordination cages based on banana-shaped ligands. Chem. Soc. Rev. 43, 1848–1860 (2014).
Zhang, G. & Mastalerz, M. Organic cage compounds—from shape-persistency to function. Chem. Soc. Rev. 43, 1934–1947 (2014).
Leenders, S. H. A. M., Gramage-Doria, R., de Bruin, B. & Reek, J. N. H. Transition metal catalysis in confined spaces. Chem. Soc. Rev. 44, 433–448 (2015).
Hof, F., Craig, S. L., Nuckolls, C. & Rebek, J. J. Molecular encapsulation. Angew. Chem. Int. Ed. 41, 1488–1508 (2002).
Rebek, J. Molecular behavior in small spaces. Acc. Chem. Res. 42, 1660–1668 (2009).
Ajami, D. & Rebek, J. More chemistry in small spaces. Acc. Chem. Res. 46, 990–999 (2012).
Ajami, D., Liu, L. & Rebek, J. Jr Soft templates in encapsulation complexes. Chem. Soc. Rev. 44, 490–499 (2015).
Jordan, J. H. & Gibb, B. C. Molecular containers assembled through the hydrophobic effect. Chem. Soc. Rev. 44, 547–585 (2014).
MacGillivray, L. R. & Atwood, J. L. A chiral spherical molecular assembly held together by 60 hydrogen bonds. Nature 389, 469–472 (1997).
Zhang, Q. & Tiefenbacher, K. Hexameric resorcinarene capsule is a Brønsted acid: investigation and application to synthesis and catalysis. J. Am. Chem. Soc. 135, 16213–16219 (2013).
Bianchini, G., La Sorella, G., Canever, N., Scarso, A. & Strukul, G. Efficient isonitrile hydration through encapsulation within a hexameric self-assembled capsule and selective inhibition by a photo-controllable competitive guest. Chem. Commun. 49, 5322–5324 (2013).
Shivanyuk, A. & Rebek, J. Reversible encapsulation by self-assembling resorcinarene subunits. Proc. Natl Acad. Sci. USA 98, 7662–7665 (2001).
Avram, L. & Cohen, Y. Spontaneous formation of hexameric resorcinarene capsule in chloroform solution as detected by diffusion NMR. J. Am. Chem. Soc. 124, 15148–15149 (2002).
Zhang, Q. & Tiefenbacher, K. Terpene cyclization catalysed inside a self-assembled cavity. Nat. Chem. 7, 197–202 (2015).
Zhang, Q., Catti, L., Pleiss, J. & Tiefenbacher, K. Terpene cyclizations inside a supramolecular catalyst: leaving-group-controlled product selectivity and mechanistic studies. J. Am. Chem. Soc. 139, 11482–11492 (2017).
Snyder, S. A., Treitler, D. S. & Brucks, A. P. Simple reagents for direct halonium-induced polyene cyclizations. J. Am. Chem. Soc. 132, 14303–14314 (2010).
Steele, C. L., Crock, J., Bohlmann, J. & Croteau, R. Sesquiterpene synthases from grand fir (Abies grandis): comparison of constitutive and wound-induced activities, and cDNA isolation, characterization, and bacterial expression of δ-selinene synthase and γ-humulene synthase. J. Biol. Chem. 273, 2078–2089 (1998).
Tanimoto, H., Kiyota, H., Oritani, T. & Matsumoto, K. Stereochemistry of a unique tricarbocyclic compound prepared by superacid-catalyzed cyclization. Synlett 1, 121–122 (1997).
Fráter, G., Müller, U. & Kraft, P. Synthesis of tricyclic ketones with sesquiterpene skeletons by acid-catalyzed rearrangement of β-monocyclofarnesol. Helv. Chim. Acta 82, 522–530 (1999).
Croteau, R., Satterwhite, D. M., Cane, D. E. & Chang, C. C. Biosynthesis of monoterpenes. Enantioselectivity in the enzymatic cyclization of (+)- and (–)-linalyl pyrophosphate to (+)- and (–)-pinene and (+)- and (–)-camphene. J. Biol. Chem. 263, 10063–10071 (1988).
Meguro, A. et al. An unusual terpene cyclization mechanism involving a carbon–carbon bond rearrangement. Angew. Chem. Int. Ed. 54, 4353–4356 (2015).
Rabe, P. et al. Mechanistic investigations of two bacterial diterpene cyclases: spiroviolene synthase and tsukubadiene synthase. Angew. Chem. Int. Ed. 56, 2776–2779 (2017).
Rabe, P. et al. Conformational analysis, thermal rearrangement, and EI-MS fragmentation mechanism of (1(10)E,4E,6S,7R)-germacradien-6-ol by 13C-labeling experiments. Angew. Chem. Int. Ed. 54, 13448–13451 (2015).
Matsuda, S. P. T., Wilson, W. K. & Xiong, Q. Mechanistic insights into triterpene synthesis from quantum mechanical calculations. Detection of systematic errors in B3LYP cyclization energies. Org. Biomol. Chem. 4, 530–543 (2006).
Tantillo, D. J. Biosynthesis via carbocations: theoretical studies on terpene formation. Nat. Prod. Rep. 28, 1035–1053 (2011).
Shimomura, O. & Johnson, F. H. The structure of Latia luciferin. Biochemistry 7, 1734–1738 (1968).
Wendt, K. U. & Schulz, G. E. Isoprenoid biosynthesis: manifold chemistry catalyzed by similar enzymes. Structure 6, 127–133 (1998).
Peters, R. J. Two rings in them all: the labdane-related diterpenoids. Nat. Prod. Rep. 27, 1521–1530 (2010).
Ghosal, M., Karpha, T. K., Pal, S. K. & Mukherjee, D. Facile synthesis of (±)-isolongifolene and (±)-isolongifolenedione involving Ar1-5 cyclisations. Tetrahedron Lett. 36, 2527–2528 (1995).
Zhang, A., Klun, J. A., Wang, S., Carroll, J. F. & Debboun, M. Isolongifolenone: a novel sesquiterpene repellent of ticks and mosquitoes. J. Med. Entomol. 46, 100–106 (2009).
Frisch, M. J. et al. Gaussian 16. Rev. A.03 (2016).
Becke, A. D. Density‐functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Adamo, C. & Barone, V. Exchange functionals with improved long-range behavior and adiabatic connection methods without adjustable parameters: the mPW and mPW1PW models. J. Chem. Phys. 108, 664–675 (1998).
Acknowledgements
This work was supported by funding from the European Research Council Horizon 2020 Programme (ERC Starting Grant 714620-TERPENECAT), Swiss National Science Foundation (as part of the NCCR Molecular Systems Engineering) and Bayerische Akademie der Wissenschaften (Junges Kolleg). We thank the computing center of the University of Cologne (RRZK) for providing CPU time on the DFG-funded supercomputer CHEOPS.
Author information
Authors and Affiliations
Contributions
K.T. conceived and supervised the project. K.T. and Q.Z. planned the project. Q.Z. carried out all the experiments except the synthesis of 13C-labelled substrates, which were synthesized by J.R. J.S.D. conceived the investigations concerning the 13C-labelled substrates. The 13C-labelled products were analysed by J.S.D. and J.R., who elucidated the proposed mechanism for the formation of isolongifolene. B.G. performed the DFT calculations. Q.Z. and K.T. compiled the first draft of the manuscript. All authors contributed to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Discussion, Supplementary Figures 1–31, Supplementary Tables 1–10, Supplementary References
Supplementary Data
Cartesian Coordinates of the optimized intermediates and transition states
Rights and permissions
About this article
Cite this article
Zhang, Q., Rinkel, J., Goldfuss, B. et al. Sesquiterpene cyclizations catalysed inside the resorcinarene capsule and application in the short synthesis of isolongifolene and isolongifolenone. Nat Catal 1, 609–615 (2018). https://doi.org/10.1038/s41929-018-0115-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-018-0115-4
This article is cited by
-
Computational prediction of complex cationic rearrangement outcomes
Nature (2023)
-
Isolation, (bio)synthetic studies and evaluation of antimicrobial properties of drimenol-type sesquiterpenes of Termitomyces fungi
Communications Chemistry (2023)
-
Biomimetic tail-to-head terpene cyclizations using the resorcin[4]arene capsule catalyst
Nature Protocols (2023)
-
Post-synthetic metalation of organic cage for enhanced porosity and catalytic performance
Science China Chemistry (2023)
-
A curved host and second guest cooperatively inhibit the dynamic motion of corannulene
Nature Communications (2021)