Abstract
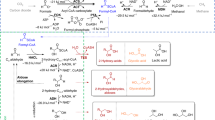
The use of CO2 as a building block for the synthesis of bulk chemicals appears highly attractive but has not been realized in industrial biotechnology due to the complexity and costly energy balance of conventional anabolic biosynthesis. Here, we describe the biocatalytic preparation of l-methionine from the abundant industrial intermediate methional under direct incorporation of CO2 by reversing the catabolic Ehrlich pathway. Despite unfavourable chemical equilibrium (1/554 M−1), the decarboxylase KdcA revealed half-maximal activity for its reverse reaction at astonishingly low CO2 pressure (320 kPa). Accordingly, it was possible to synthesize l-methionine under a 2 bar CO2 atmosphere when coupled to an energetically favourable transaminase or amino acid dehydrogenase reaction. Similarly, l-leucine and l-isoleucine were prepared via biocatalytic carboxylation of 3- or 2-methylbutanal, respectively. Our findings open a biotechnological route towards industrial products and enable further syntheses involving the fixation of gaseous CO2 by simply applying decarboxylases in the reverse mode.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Beckman, E. J. Sustainable chemistry: putting carbon dioxide to work. Nature 531, 180–181 (2016).
Scott, A. Learning to love CO2. Chem. Eng. News 93, 10–16 (2015).
Haas, T., Krause, R., Weber, R., Demler, M. & Schmid, G. Technical photosynthesis involving CO2 electrolysis and fermentation. Nat. Catal. 1, 32–39 (2018).
Glueck, S. M., Gumus, S., Fabian, W. M. & Faber, K. Biocatalytic carboxylation. Chem. Soc. Rev. 39, 313–328 (2010).
Wieser, M., Fujii, N., Yoshida, T. & Nagasawa, T. Carbon dioxide fixation by reversible pyrrole-2-carboxylate decarboxylase from Bacillus megaterium PYR2910. Eur. J. Biochem. 257, 495–499 (1998).
Jitrapakdee, S. et al. Structure, mechanism and regulation of pyruvate carboxylase. Biochem. J. 413, 369–387 (2008).
Schwander, T. et al. A synthetic pathway for the fixation of carbon dioxide in vitro. Science 354, 900–904 (2016).
Bar-Even, A., Noor, E., Lewis, N. E. & Milo, R. Design and analysis of synthetic carbon fixation pathways. Proc. Natl Acad. Sci. USA 107, 8889–8894 (2010).
Hazelwood, L. A., Daran, J. M., van Maris, A. J., Pronk, J. T. & Dickinson, J. R. The Ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 74, 2259–2266 (2008).
Willke, T. Methionine production—a critical review. Appl. Microbiol. Biotechnol. 98, 9893–9914 (2014).
Killenberg-Jabs, M., König, S., Eberhardt, I., Hohmann, S. & Hübner, G. Role of Glu51 for cofactor binding and catalytic activity in pyruvate decarboxylase from yeast studied by site-directed mutagenesis. Biochemistry 36, 1900–1905 (1997).
Kneen, M. M. et al. Characterization of a thiamin diphosphate-dependent phenylpyruvate decarboxylase from Saccharomyces cerevisiae. FEBS J. 278, 1842–1853 (2011).
Smit, B. A. et al. Identification, cloning, and characterization of a Lactococcus lactis branched-chain α-keto acid decarboxylase involved in flavor formation. Appl. Environ. Microbiol. 71, 303–311 (2005).
Dolzan, M. et al. Crystal structure and reactivity of YbdL from Escherichia coli identify a methionine aminotransferase function. FEBS Lett. 571, 141–146 (2004).
Miyazaki, M., Shibue, M., Ogino, K., Nakamura, H. & Maeda, H. Enzymatic synthesis of pyruvic acid from acetaldehyde and carbon dioxide. Chem. Commun. 18, 1800–1801 (2001).
Tong, X., El-Zahab, B., Zhao, X., Liu, Y. & Wang, P. Enzymatic synthesis of l-lactic acid from carbon dioxide and ethanol with an inherent cofactor regeneration cycle. Biotechnol. Bioeng. 108, 465–469 (2011).
Wichmann, R., Wandrey, C., Buckmann, A. F. & Kula, M. R. Continuous enzymatic transformation in an enzyme membrane reactor with simultaneous NAD(H) regeneration. Biotechnol. Bioeng. 23, 2789–2802 (1981).
Li, H. et al. Cloning, protein sequence clarification, and substrate specificity of a leucine dehydrogenase from Bacillus sphaericus ATCC4525. Appl. Biochem. Biotechnol. 158, 343–351 (2009).
Hillmann, H. & Hofmann, T. Quantitation of key tastants and re-engineering the taste of parmesan cheese. J. Agric. Food Chem. 64, 1794–1805 (2016).
Takada, H., Yoshimura, T., Ohshima, T., Esaki, N. & Soda, K. Thermostable phenylalanine dehydrogenase of Thermoactinomyces intermedius: cloning, expression, and sequencing of its gene. J. Biochem. 109, 371–376 (1991).
Andrews, F. H. & McLeish, M. J. Substrate specificity in thiamin diphosphate-dependent decarboxylases. Bioorg. Chem. 43, 26–36 (2012).
Cleland, W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim. Biophys. Acta 67, 104–137 (1963).
Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 15, 4399–4981 (2015).
Lerchner, A., Achatz, S., Rausch, C., Haas, T. & Skerra, A. Coupled enzymatic alcohol-to-amine conversion of isosorbide using engineered transaminases and dehydrogenases. ChemCatChem 5, 3374–3383 (2013).
Jensen, K. F. The Escherichia coli K-12 “wild types” W3110 and MG1655 have an rph frameshift mutation that leads to pyrimidine starvation due to low pyrE expression levels. J. Bacteriol. 175, 3401–3407 (1993).
Studier, F. W. & Moffatt, B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189, 113–130 (1986).
Skerra, A. Use of the tetracycline promoter for the tightly regulated production of a murine antibody fragment in Escherichia coli. Gene 151, 131–135 (1994).
Acknowledgements
The authors thank Evonik Industries for financial support, and T. Haas and H. Jakob for fruitful discussions. They are also grateful to C. Schmid, C. Dawid and T. Hofmann at TUM for LC-MS/MS measurements.
Author information
Authors and Affiliations
Contributions
A.S. developed the concept of the study. J.M. and L.E. contributed to planning the experiments, and performed the measurements. All authors conducted data analysis and discussed the results. J.M. and A.S. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
A.S. and L.E. declare inventorship on a patent application claiming subject matter of this study (WO 2018/104143 A1).
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–7 and Supplementary Notes 1 & 2
Rights and permissions
About this article
Cite this article
Martin, J., Eisoldt, L. & Skerra, A. Fixation of gaseous CO2 by reversing a decarboxylase for the biocatalytic synthesis of the essential amino acid l-methionine. Nat Catal 1, 555–561 (2018). https://doi.org/10.1038/s41929-018-0107-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-018-0107-4
This article is cited by
-
Harnessing transaminases to construct azacyclic non-canonical amino acids
Nature Synthesis (2024)
-
A roadmap towards integrated catalytic systems of the future
Nature Catalysis (2020)