Abstract
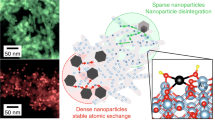
Supported metal nanoparticle catalysts are widely used in industry but suffer from deactivation resulting from metal sintering and coke deposition at high reaction temperatures. Here, we show an efficient and general strategy for the preparation of supported metal nanoparticle catalysts with very high resistance to sintering by fixing the metal nanoparticles (platinum, palladium, rhodium and silver) with diameters in the range of industrial catalysts (0.8–3.6 nm) within zeolite crystals (metal@zeolite) by means of a controllable seed-directed growth technique. The resulting materials are sinter resistant at 600–700 °C, and the uniform zeolite micropores allow for the diffusion of reactants enabling contact with the metal nanoparticles. The metal@zeolite catalysts exhibit long reaction lifetimes, outperforming conventional supported metal catalysts and commercial catalysts consisting of metal nanoparticles on the surfaces of solid supports during the catalytic conversion of C1 molecules, including the water-gas shift reaction, CO oxidation, oxidative reforming of methane and CO2 hydrogenation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ertl, G., Knözinger, H., Schüth, F. & Weitkamp, J. Handbook of Heterogeneous Catalysis (Wiley, Weinheim, 2008).
Behrens, M. et al. The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts. Science 336, 893–897 (2012).
Lu, J. et al. Coking- and sintering-resistant palladium catalysts achieved through atomic layer deposition. Science 335, 1205–1208 (2012).
Li, W.-Z. et al. Stable platinum nanoparticles on specific MgAl2O4 spinel facets at high temperatures in oxidizing atmospheres. Nat. Chem. 4, 2481 (2013).
Prieto, G., Zečević, J., Friedrich, H., de Jong, K. P. & de Jongh, P. E. Towards stable catalysts by controlling collective properties of supported metal nanoparticles. Nat. Mater. 12, 34–39 (2013).
Tang, H. et al. Strong metal–support interactions between gold nanoparticles and nonoxides. J. Am. Chem. Soc. 138, 56–59 (2016).
Ta, N. et al. Stabilized gold nanoparticles on ceria nanorods by strong interfacial anchoring. J. Am. Chem. Soc. 134, 20585–20588 (2012).
Zhao, M.-Q. et al. Embedded high density metal nanoparticles with extraordinary thermal stability derived from guest-host mediated layered double hydroxides. J. Am. Chem. Soc. 132, 14739–14741 (2010).
Tang, H. et al. Ultrastable hydroxyapatite/titanium-dioxide-supported gold nanocatalyst with strong metal–support interaction for carbon monoxide oxidation. Angew. Chem. Int. Ed. 55, 10606–10611 (2016).
Sattler, J. J. H. B. et al. Platinum-promoted Ga/Al2O3 as highly active, selective, and stable catalyst for the dehydrogenation of propane. Angew. Chem. Int. Ed. 53, 9251–9256 (2014).
Zhang, L. Y. et al. Stabilization of palladium nanoparticles on nanodiamond–graphene core–shell supports for CO oxidation. Angew. Chem. Int. Ed. 54, 15823–15826 (2015).
Shi, L. et al. Al2O3 nanosheets rich in pentacoordinate Al3+ ions stabilize Pt–Sn clusters for propane dehydrogenation. Angew. Chem. Int. Ed. 54, 13994–13998 (2015).
Wang, S. et al. Aggregation-free gold nanoparticles in ordered mesoporous carbons: toward highly active and stable heterogeneous catalysts. J. Am. Chem. Soc. 135, 11849–11860 (2013).
Zhou, H. P. et al. Thermally stable Pt/CeO2 hetero-nanocomposites with high catalytic activity. J. Am. Chem. Soc. 132, 4998–4999 (2010).
Farrauto, R. J. & Bartholomew, C. H. Fundamentals of Industrial Catalytic Process (Blackie, London, 1997).
Morgan, K., Goguet, A. & Hardacre, C. Metal redispersion strategies for recycling of supported metal catalysts: a perspective. ACS Catal. 5, 3430–3445 (2015).
Dick, K., Dhanasekaran, T., Zhang, Z. Y. & Meisel, D. Size-dependent melting of silica-encapsulated gold nanoparticles. J. Am. Chem. Soc. 124, 2312–2317 (2002).
Joo, S. H. et al. Thermally stable Pt/mesoporous silica core–shell nanocatalysts for high-temperature reactions. Nat. Mater. 8, 126–131 (2009).
Yu, K., Wu, Z. C., Zhao, Q. R., Li, B. X. & Xie, Y. High-temperature-stable Au@SnO2 core/shell supported catalyst for CO oxidation. J. Phys. Chem. C 112, 2244–2247 (2008).
Cargnello, M. et al. Exceptional activity for methane combustion over modular Pd@CeO2 subunits on functionalized Al2O3. Science 337, 713–717 (2012).
Arnal, P. M., Comotti, M. & Schüth, F. High-temperature-stable catalysts by hollow sphere encapsulation. Angew. Chem. Int. Ed. 45, 8224–8227 (2006).
O’Neill, B. J. et al. Stabilization of copper catalysts for liquid-phase reactions by atomic layer deposition. Angew. Chem. Int. Ed. 52, 13808–13812 (2013).
Zhan, W. et al. A sacrificial coating strategy toward enhancement of metal–support interaction for ultrastable Au nanocatalysts. J. Am. Chem. Soc. 138, 16130–16139 (2016).
Huang, W. et al. Low-temperature transformation of methane to methanol on Pd1O4 single sites anchored on the internal surface of microporous silicate. Angew. Chem., Int. Ed. 55, 13441–13445 (2016).
Laursen, A. B. et al. Substrate size-selective catalysis with zeolite-encapsulated gold nanoparticles. Angew. Chem. Int. Ed. 49, 3504–3507 (2010).
Goel, S., Wu, Z., Zones, S. I. & Iglesia, E. Synthesis and catalytic properties of metal clusters encapsulated within small-pore (SOD, GIS, ANA) zeolites. J. Am. Chem. Soc. 134, 17688–17695 (2012).
Wang, N. et al. In situ confinement of ultrasmall Pd clusters within nanosized silicalite-1 zeolite for highly efficient catalysis of hydrogen generation. J. Am. Chem. Soc. 138, 7484–7487 (2016).
Liu, L. et al. Generation of subnanometric platinum with high stability during transformation of a 2D zeolite into 3D. Nat. Mater. 16, 132–138 (2017).
Bond, G. C., Louis, C. & Thompson, D. T. Catalysis by Gold (Imperial College Press, London, 2006).
Iglesias-Juez, A., Kubacka, A., Fernández-García, M., Michiel, M. D. & Newton, M. A. Nanoparticulate Pd supported catalysts: size-dependent formation of Pd(I)/Pd(0) and their role in CO elimination. J. Am. Chem. Soc. 133, 4484–4489 (2011).
Xiao, F. S., Weber, W. A., Alexeev, O. & Gates, B. C. Probing the limits of structure insensitivity: size-dependent catalytic activity of Al2O3-supported iridium clusters and particles for toluene hydrogenation. Stud. Surf. Sci. Catal. 101, 1135–1144 (1996).
Kistler, J. D. et al. A single-site platinum CO oxidation catalyst in zeolite KLTL: microscopic and spectroscopic determination of the locations of the platinum atoms. Angew. Chem. Int. Ed. 53, 8904–8907 (2014).
Cui, T.-L. et al. Encapsulating palladium nanoparticles inside mesoporous MFI zeolite nanocrystals for shape-selective catalysis. Angew. Chem. Int. Ed. 55, 9178–9182 (2016).
Wang, C. et al. Product selectivity controlled by zeolite crystals in biomass hydrogenation over a palladium catalyst. J. Am. Chem. Soc. 138, 7880–7883 (2016).
Sachtler, W. M. H. Metal clusters in zeolites: an intriguing class of catalysts. Acc. Chem. Res. 26, 383–387 (1993).
Sun, T. & Seff, K. Silver clusters and chemistry in zeolites. Chem. Rev. 94, 857–870 (1994).
Guzman, J. in Model Systems in Catalysis (ed. Rioux, R.) Ch. 19 (Springer, New York, 2010).
Creyghton, E. J. & Downing, R. S. Shape-selective hydrogenation and hydrogen transfer reactions over zeolite catalysts. J. Mol. Catal. A 134, 47–61 (1998).
Alexeev, O. S. & Gates, B. C. Supported bimetallic cluster catalysts. Ind. Eng. Chem. Res. 42, 1571–1587 (2003).
Yamamoto, T., Shido, T., Inagaki, S., Fukushima, Y. & Ichikawa, M. Ship-in-bottle synthesis of [Pt15(CO)30]2– encapsulated in ordered hexagonal mesoporous channels of FSM-16 and their effective catalysis in water-gas shift reaction. J. Am. Chem. Soc. 118, 5810–5811 (1996).
Rodriguez, J. A. et al. Activity of CeOx and TiOx nanoparticles grown on Au(111) in the water-gas shift reaction. Science 318, 1757–1760 (2007).
Yang, M. et al. Catalytically active Au–O(OH)x– species stabilized by alkali ions on zeolites and mesoporous oxides. Science 346, 1498–1501 (2014).
Yang, M. et al. A common single-site Pt(II)–O(OH)x– species stabilized by sodium on “active” and “inert” supports catalyzes the water-gas shift reaction. J. Am. Chem. Soc. 137, 3470–3473 (2015).
Chen, M. S., Cai, Y., Yan, Z. & Goodman, D. W. On the origin of the unique properties of supported Au nanoparticles. J. Am. Chem. Soc. 128, 6341–6346 (2006).
Ouyang, R. & Li, W.-X. Adsorbed CO induced change of the adsorption site and charge of Au adatoms on FeO(111)/Ru(0001). Chin. J. Catal. 34, 1820–1825 (2013).
Fu, Q. et al. Interface-confined ferrous centers for catalytic oxidation. Science 328, 1141–1144 (2010).
Chen, G. et al. Interfacial effects in iron–nickel hydroxide–platinum nanoparticles enhance catalytic oxidation. Science 344, 495–499 (2014).
Hickman, D. A. & Schmidt, L. D. Production of syngas by direct catalytic oxidation of methane. Science 259, 343–346 (1993).
Ayabe, S. et al. Catalytic autothermal reforming of methane and propane over supported metal catalysts. Appl. Catal. A 241, 261–269 (2003).
Carrasquillo-Flores, R. et al. Reverse water-gas shift on interfacial sites formed by deposition of oxidized molybdenum moieties onto gold nanoparticles. J. Am. Chem. Soc. 137, 10317–10325 (2015).
Acknowledgements
This work is supported by the National Key Research and Development Program of China (2018YFB060128) and National Natural Science Foundation of China (91645105, 91634201 and 21720102001). L.W. gratefully acknowledges the Natural Science Foundation of Zhejiang Province (LR18B030002). B.C.G. acknowledges financial support from the US Department of Energy, Office of Science, Basic Energy Sciences (grant DE-FG02-04ER15513). H.Z. acknowledges financial support from the Carl-Zeiss-Stiftung. The work reported in this paper is protected by Chinese patents (application numbers 201610342078.0 and 201610341082.5).
Author information
Authors and Affiliations
Contributions
J.Z. performed the catalyst preparation, characterizations and catalytic tests. G.W. and C.W. performed the catalytic tests. B.Z., D.S.S., H.Z., U.K., Y.Z., L.L. and Y.H. performed the TEM characterization. B.C.G. performed the data analysis and offered helpful suggestions. L.W. and F.-S.X. designed this study, analysed the data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods; Supplementary Figures 1–54; Supplementary Table 1; Supplementary Notes 1–4
Rights and permissions
About this article
Cite this article
Zhang, J., Wang, L., Zhang, B. et al. Sinter-resistant metal nanoparticle catalysts achieved by immobilization within zeolite crystals via seed-directed growth. Nat Catal 1, 540–546 (2018). https://doi.org/10.1038/s41929-018-0098-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-018-0098-1
This article is cited by
-
Generating active metal/oxide reverse interfaces through coordinated migration of single atoms
Nature Communications (2024)
-
Synthesis of core@shell catalysts guided by Tammann temperature
Nature Communications (2024)
-
Co-aromatization of methane and hexane over Pt encapsulated in ZSM-5 zeolite and the electronic effect of K promoters
Science China Chemistry (2024)
-
Nanoengineered approaches to improve the efficacy of targeted drug delivery for the treatment of malignancy: a comprehensive review
Future Journal of Pharmaceutical Sciences (2023)
-
Germanium-enriched double-four-membered-ring units inducing zeolite-confined subnanometric Pt clusters for efficient propane dehydrogenation
Nature Catalysis (2023)