Abstract
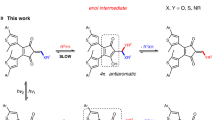
The fundamental properties of a polymeric material are ultimately governed by its structure, which mainly relies on monomer composition and connection, topology, chain length, and polydispersity. Thus far, these structural characteristics are typically set ex situ by the specific polymerization procedure, eventually limiting the future design space for the creation of more sophisticated polymers. Herein, we report on a single photoswitchable catalyst system, which enables in situ remote control over the ring-opening polymerization of l-lactide and further allows regulation of the incorporation of trimethylene carbonate and δ-valerolactone monomers in copolymerizations. By implementing a phenol moiety into a diarylethene-type structure, we exploit light-induced keto–enol tautomerism to switch the hydrogen-bonding-mediated monomer activation reversibly ON and OFF. This general and versatile principle allows for exquisite external modulation of ground-state catalysis of a living polymerization process in a closed system by ultraviolet and visible light and should thereby facilitate the generation of new polymer structures.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Leibfarth, F. A., Mattson, K. M., Fors, B. P., Collins, H. A. & Hawker, C. J. External regulation of controlled polymerizations. Angew. Chem. Int. Ed. 52, 199–210 (2013).
Aida, T., Meijer, E. W. & Stupp, S. I. Functional supramolecular polymers. Science 335, 813–817 (2012).
Kamber, N. E. et al. Organocatalytic ring-opening polymerization. Chem. Rev. 107, 5813–5840 (2007).
Kiesewetter, M. K., Shin, E. J., Hedrick, J. L. & Waymouth, R. M. Organocatalysis: opportunities and challenges for polymer synthesis. Macromolecules 43, 2093–2107 (2010).
Thomas, M. C. Stereocontrolled ring-opening polymerization of cyclic esters: synthesis of new polyester microstructures. Chem. Soc. Rev. 39, 165–173 (2010).
Thomas, C. & Bibal, B. Hydrogen-bonding organocatalysts for ring-opening polymerization. Green Chem. 16, 1687–1699 (2014).
Eisenreich, F., Viehmann, P., Müller, F. & Hecht, S. Electronic activity tuning of acyclic guanidines for lactide polymerization. Macromolecules 48, 8729–8732 (2015).
Zhang, X., Jones, G. O., Hedrick, J. L. & Waymouth, R. M. Fast and selective ring-opening polymerizations by alkoxides and thioureas. Nat. Chem. 8, 1047–1053 (2016).
Teator, A. J., Lastovickova, D. N. & Bielawski, C. W. Switchable polymerization catalysts. Chem. Rev. 116, 1969–1992 (2016).
Gregson, C. K. A. et al. Redox control within single-site polymerization catalysts. J. Am. Chem. Soc. 128, 7410–7411 (2006).
Wang, X. et al. Redox control of group 4 metal ring-opening polymerization activity toward l-lactide and ε-caprolactone. J. Am. Chem. Soc. 136, 11264–11267 (2014).
Quan, S. M., Wang, W., Zhang, R. & Diaconescu, P. L. Redox switchable copolymerization of cyclic esters and epoxides by a zirconium complex. Macromolecules 49, 6768–6778 (2016).
Biernesser, A. B., Li, B. & Byers, J. A. Redox-controlled polymerization of lactide catalyzed by bis(imino)pyridine iron bis(alkoxide) complexes. J. Am. Chem. Soc. 135, 16553–16560 (2013).
Biernesser, A. B., Delle Chiaie, K. R., Curley, J. B. & Byers, J. A. Redox copolymerization of lactide and an epoxide facilitated by a redox switchable iron-based catalyst. Angew. Chem. Int. Ed. 55, 5251–5254 (2016).
Delle Chiaie, K. R. et al. Redox-triggered crosslinking of a degradable polymer. Polym. Chem. 7, 4675–4681 (2016).
Zhu, Y., Romain, C. & Williams, C. K. Selective polymerization catalysis: controlling the metal chain end group to prepare block copolyesters. J. Am. Chem. Soc. 137, 12179–12182 (2015).
Romain, C. et al. Chemoselective polymerizations from mixtures of epoxide, lactone, anhydride, and carbon dioxide. J. Am. Chem. Soc. 138, 4120–4131 (2016).
Göstl, R., Senf, A. & Hecht, S. Remote-controlling chemical reactions by light: towards chemistry with high spatio-temporal resolution. Chem. Soc. Rev. 43, 1982–1996 2014).
Bratton, D., Yang, D., Dai, J. & Ober, C. K. Recent progress in high resolution lithography. Polym. Adv. Technol. 17, 94–103 (2006).
Tumbleston, J. R. et al. Continuous liquid interface production of 3D objects. Science 347, 1349–1352 (2015).
Fors, B. P. & Hawker, C. J. Control of a living radical polymerization of methacrylates by light. Angew. Chem. Int. Ed. 51, 8850–8853 (2012).
Kottisch, V., Michaudel, Q. & Fors, B. P. Cationic polymerization of vinyl ethers controlled by visible light. J. Am. Chem. Soc. 138, 15535–15538 (2016).
Stoll, R. S. & Hecht, S. Artificial light-gated catalyst systems. Angew. Chem. Int. Ed. 49, 5054–5075 (2010).
Neilson, B. M. & Bielawski, C. W. Illuminating photoswitchable catalysis. ACS Catal. 3, 1874–1885 (2013).
Vlatković, M, Collins, B. S. L. & Feringa, B. L. Dynamic responsive systems for catalytic function. Chem. Eur. J. 22, 17080–17111 (2016).
Blanco, V., Leigh, D. A. & Marcosa, V. Artificial switchable catalysts. Chem. Soc. Rev. 44, 5341–5370 (2015).
Würthner, F. & Rebek, J.Jr. Light-switchable catalysis in synthetic receptors. Angew. Chem. Int. Ed. 34, 446–448 (1995).
Cacciapaglia, R., Di Stefano, S. & Mandolini, L. The bis–barium complex of a butterfly crown ether as a phototunable supramolecular catalyst. J. Am. Chem. Soc. 125, 2224–2227 (2003).
Sud, D., Norsten, T. B. & Branda, N. R. Photoswitching of stereoselectivity in catalysis using a copper dithienylethene complex. Angew. Chem. Int. Ed. 44, 2019–2021 (2005).
Stoll, R. S. et al. Photoswitchable catalysts: correlating structure and conformational dynamics with reactivity by a combined experimental and computational approach. J. Am. Chem. Soc. 131, 357–367 (2009).
Wei, Y., Han, S., Kim, J., Soh, S. & Grzybowski, B. A. Photoswitchable catalysis mediated by dynamic aggregation of nanoparticles. J. Am. Chem. Soc. 132, 11018–11020 (2010).
Wang, J. & Feringa, B. L. Dynamic control of chiral space in a catalytic asymmetric reaction using a molecular motor. Science 331, 1429–1432 (2011).
Berryman, O. B., Sather, A. C., Lledó, A. & Rebek, J. Switchable catalysis with a light-responsive cavitand. Angew. Chem. Int. Ed. 50, 9400–9403 (2011).
Wilson, D. & Branda, N. R. Turning “on” and “off” a pyridoxal 5′-phosphate mimic using light. Angew. Chem. Int. Ed. 51, 5431–5434 (2012).
Imahori, T., Yamaguchi, R. & Kurihara, S. Azobenzene-tethered bis(trityl alcohol) as a photoswitchable cooperative acid catalyst for Morita–Baylis–Hillman reactions. Chem. Eur. J. 18, 10802–10807 (2012).
Osorio-Planes, L., Rodríguez-Escrich, C. & Pericàs, M. A. Photoswitchable thioureas for the external manipulation of catalytic activity. Org. Lett. 16, 1704–1707 (2014).
Zhao, D., Neubauer, T. M. & Feringa, B. L. Dynamic control of chirality in phosphine ligands for enantioselective catalysis. Nat. Commun. 6, 6652 (2015).
Zhao, H. et al. Reversible trapping and reaction acceleration within dynamically self-assembling nanoflasks. Nat. Nanotech. 11, 82–88 (2016).
Neri, S., Garcia Martin, S., Pezzato, C. & Prins, L. J. Photoswitchable catalysis by a nanozyme mediated by a light-sensitive cofactor. J. Am. Chem. Soc. 139, 1794–1797 (2017).
Neilson, B. M. & Bielawski, C. W. Photoswitchable NHC-promoted ring-opening polymerizations. Chem. Commun. 49, 5453–5455 (2013).
Fu, C., Xu, J. & Boyer, C. Photoacid-mediated ring opening polymerization driven by visible light. Chem. Commun. 52, 7126–7129 (2016).
Dai, Z., Cui, Y., Chen, C. & Wu, J. Photoswitchable ring-opening polymerization of lactide catalyzed by azobenzene-based thiourea. Chem. Commun. 52, 8826–8829 (2016).
Teator, A. J., Shao, H., Lu, G., Liu, P. & Bielawski, C. W. A photoswitchable olefin metathesis catalyst. Organometallics 36, 490–497 (2017).
Thomas, C. et al. Phenols and tertiary amines: an amazingly simple hydrogen-bonding organocatalytic system promoting ring opening polymerization. Adv. Synth. Catal. 353, 1049–1054 (2011).
Yamaguchi, T. et al. Photochromic reaction of diarylethenes having phenol moiety as an aryl ring. Bull. Chem. Soc. Jpn 87, 528–538 (2014).
Kawai, S. H., Gilat, S. L. & Lehn, J.-M. Photochemical pKa-modulation and gated photochromic properties of a novel diarylethene switch. Eur. J. Org. Chem. 1999, 2359–2366 (1999).
Herder, M. et al. Improving the fatigue resistance of diarylethene switches. J. Am. Chem. Soc. 137, 2738–2747 (2015).
Herder, M. et al. Light-controlled reversible modulation of frontier molecular orbital energy levels in trifluoromethylated diarylethenes. Chem. Eur. J. 23, 3743–3754 (2017).
Pratt, R. C. et al. Exploration, optimization, and application of supramolecular thiourea−amine catalysts for the synthesis of lactide (co)polymers. Macromolecules 39, 7863–7871 (2006).
Todd, R., Rubio, G., Hall, D. J., Tempelaar, S. & Dove, A. P. Benzyl bispidine as an efficient replacement for (–)-sparteine in ring opening polymerisation. Chem. Sci. 4, 1092–1097 (2013).
Kowalski, A., Libiszowski, J., Duda, A. & Penczek, S. Polymerization of l,l-dilactide initiated by tin(II) butoxide. Macromolecules 33, 1964–1971 (2000).
Corrigan, N., Shanmugam, S., Xu, J. & Boyer, C. Photocatalysis in organic and polymer synthesis. Chem. Soc. Rev. 45, 6165–6212 (2016).
Palard, I., Schappacher, M., Belloncle, B., Soum, A. & Guillaume, S. Unprecedented polymerization of trimethylene carbonate initiated by a samarium borohydride complex: mechanistic insights and copolymerization with ε-caprolactone. Chem. Eur. J. 13, 1511–1521 (2007).
Save, M., Schappacher, M. & Soum, A. Controlled ring-opening polymerization of lactones and lactides initiated by lanthanum isopropoxide, 1. General aspects and kinetics. Macromol. Chem. Phys. 203, 889–899 (2002).
Acknowledgements
F.E. and M.K. are indebted to the Fonds der Chemischen Industrie and Studienstiftung des deutschen Volkes, respectively, for providing doctoral fellowships. Generous support from the European Research Council via ERC-2012-STG_308117 (Light4Function) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
F.E., M.K. and S.P.I. synthesized diarylethene 1. F.E. conducted ultraviolet visible spectroscopy and polymerization experiments, and analysed polymers via NMR and GPC measurements. F.E. and A.D. performed kinetics studies of polymerizations via NMR spectroscopy. T.S. conducted MALDI-MS measurements. B.M.S. solved the single-crystal X-ray structure. F.E., M.K. and S.H. conceived the idea, designed the study and wrote the manuscript. All authors discussed the results and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Figures 1–48, Supplementary References
Rights and permissions
About this article
Cite this article
Eisenreich, F., Kathan, M., Dallmann, A. et al. A photoswitchable catalyst system for remote-controlled (co)polymerization in situ. Nat Catal 1, 516–522 (2018). https://doi.org/10.1038/s41929-018-0091-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-018-0091-8
This article is cited by
-
Spatiotemporal control for integrated catalysis
Nature Reviews Methods Primers (2023)
-
A catalytically active oscillator made from small organic molecules
Nature (2023)
-
Diblock dialternating terpolymers by one-step/one-pot highly selective organocatalytic multimonomer polymerization
Nature Communications (2021)
-
Macrocycles in dual role: ancillary ligands in metal complexes and organocatalysts for the ring-opening polymerization of lactide
Journal of Inclusion Phenomena and Macrocyclic Chemistry (2021)
-
Towards artificial molecular factories from framework-embedded molecular machines
Nature Reviews Chemistry (2020)