Abstract
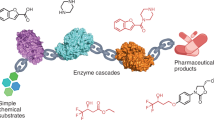
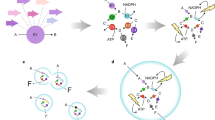
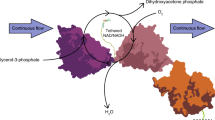
The synthesis of alcohols from amine starting materials is an excellent yet challenging strategy for the preparation of pharmaceuticals and polymers. Here we developed a versatile, self-sustaining closed-loop multienzymatic platform for the biocatalytic synthesis of a large range of non-commercially available products in a continuous flow with excellent yields (80 to >99%), reaction times and optical purity of secondary alcohols (>99 enantiomeric excess). This process was also extended to the conversion of biogenic amines into high-value alcohols, such as the powerful antioxidant hydroxytyrosol, and the synthesis of enantiopure 2-arylpropanols via the dynamic kinetic resolution of commercially affordable racemic amines. The system exploits the in situ immobilization of transaminases and redox enzymes which were combined to cater for a fully automated, ultra-efficient synthetic platform with cofactor recycling, in-line recovery of benign by-products and recirculation of the aqueous media that contains the recycled cofactors in catalytic amounts, which increases the efficiency of the system by over 20-fold.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bartoszewicz, A., Ahlsten, N. & Martin-Matute, B. Enantioselective synthesis of alcohols and amines by iridium-catalysed hydrogenation, transfer hydrogenation, and related processes. Chem. Eur. J. 19, 7274–7302 (2013).
Ahn, Y., Ko, S. B., Kim, M. J. & Park, J. Racemization catalysts for the dynamic kinetic resolution of alcohols and amines. Coord. Chem. Rev. 252, 647–658 (2008).
Romano, D., Gandolfi, R., Guglielmetti, S. & Molinari, F. Enzymatic hydrolysis of capsaicins for the production of vanillylamine using ECB deacylase from Actinoplanes utahensis. Food Chem. 124, 1096–1098 (2011).
Duarte, D. R., Castillo, E., Barzana, E. & Lopez-Munguia, A. Capsaicin hydrolysis by Candida antarctica lipase. Biotechol. Lett. 22, 1811–1814 (2000).
Pouy, M. J., Stanley, L. M. & Hartwig, J. F. Enantioselective, iridium-catalysed monoallylation of ammonia. J. Am. Chem. Soc. 131, 11312–11313 (2009).
Imm, S., Bähn, S., Neubert, L., Neumann, H. & Beller, M. An efficient and general synthesis of primary amines by ruthenium-catalysed amination of secondary alcohols with ammonia. Angew. Chem. Int. Ed. 122, 8303–8306 (2010).
Mutti, F., Knaus, T., Scrutton, N. S., Breuer, M. & Turner, N. J. Conversion of alcohols to enantiopure amines through dual-enzyme hydrogen-borrowing cascades. Science 349, 1525–1529 (2015).
Baumgarten, R. J. Aliphatic deaminations in organic synthesis. J. Chem. Educ. 43, 398–408 (1966).
Rahman, S. M. A., Ohno, H., Maezaki, N., Iwata, C. & Tanaka, T. Total synthesis of (±)-scopadulin. Org. Lett. 2, 2893–2895 (2000).
Kanbara, Y., Abe, T., Fushimi, N. & Ikeno, T. Base-catalysed direct transformation of benzylamines into benzyl alcohols. Synlett 23, 706–710 (2012).
Carlquist P. M., Gorwa M. F. & Weber, N. Stereoselective biosynthesis in microbial host cells. World Intellectual Property Organization patent 2014/0376376 A1 (2014).
Khusnutdinova, J. R., Ben-David, Y. & Milstein, D. Direct deamination of primary amines by water to produce alcohols. Angew. Chem. Int. Ed. 52, 6269–6272 (2013).
Sadri, A.S. & Fiksdahl, A. Stereoselective transformation of amines via chiral 2,4,6-triphenylpyridinium intermediates. Tetrahedron: Asymmetry 12, 1947–1951 (2001).
White, E. H., Li, M. & Lu, S. N-nitrososulfamates: sources of carbonium ions in aqueous media and substrates in solid-state decompositions. J. Org. Chem. 57, 1252–1258 (1992).
Elswort, J. D. & Roth, R. H. Dopamine synthesis, uptake, metabolism, and receptors: relevance to gene therapy of Parkinson's disease. Exp. Neurol. 144, 4–9 (1997).
Bouton, A. A. & Eisenhofer, G. Catecholamine metabolism: from molecular understanding to clinical diagnosis and treatment. Adv. Pharmacol. 42, 273–292 (1998).
Tamborini, L., Fernandes, P., Paradisi, F. & Molinari, F. Flow bioreactors as complementary tools for biocatalytic process intensification. Trends Biotechnol. 36, 73–88 (2018).
Britton, J. & Jamison, T. F. The assembly and use of continuous flow systems for chemical synthesis. Nat. Protoc. 12, 2423–2446 (2017).
Yuryev, R., Strompen, S. & Liese, A. Coupled chemo(enzymatic) reactions in continuous flow. Beilstein J. Org. Chem. 7, 1449–1467 (2011).
Ricca, E., Brucher, B. & Schrittwieser, J. H. Multi‐enzymatic cascade reactions: overview and perspectives. Adv. Synth. Catal. 353, 2239–2262 (2011).
Santacoloma, P. A., Sin, G., Gernaey, K. V. & Woodley, J. M. Multienzyme-catalysed processes: next-generation biocatalysis. Org. Process Res. Dev. 15, 203–212 (2011).
Gruber, P. et al. Fundamentals and applications of immobilised microfluidic enzymatic reactors. Chem. Technol. Biotechnol. 86, 325–334 (2011).
Lentes, J. et al. Competitive and sustainable manufacturing by means of ultra-efficient factories in urban surroundings. Int. J. Prod. Res. 55, 480–491 (2017).
Garcia-Galan, C., Berenguer-Murcia, Á., Fernandez-Lafuente, R. & Rodrigues, R. C. Potential of different enzyme immobilisation strategies to improve enzyme performance. Adv. Synth. Catal. 353, 2885–2904 (2011).
Abaházi, E. et al. Covalently immobilised Trp60Cys mutant of ω-transaminase from Chromobacterium violaceum for kinetic resolution of racemic amines in batch and continuous-flow modes. Biochem. Eng. J. 132, 270–278 (2018).
Savile, C. K. et al. Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture. Science 329, 305–309 (2010).
Monti, D., Ottolina, G., Carrea, G. & Riva, S. Redox reactions catalysed by isolated enzymes. Chem. Rev. 111, 4111–4140 (2011).
Contente, M. L., Molinari, F., Serra, I., Pinto, A. & Romano, D. Stereoselective enzymatic reduction of ethyl secodione: preparation of a key intermediate for the total synthesis of steroids. Eur. J. Org. Chem. 2016, 1260–1263 (2016).
Gomm, A. & O’Reilly, E. Transaminases for chiral amine synthesis. Curr. Opin. Chem. Biol. 43, 106–112 (2018).
Liu, W. & Wang, P. Cofactor regeneration for sustainable enzymatic biosynthesis. Biotechnol. Adv. 4, 269–384 (2007).
Schmidt, N. G., Simon, R. C. & Kroutil, W. Biocatalytic asymmetric synthesis of optically pure aromatic propargylic amines employing ω‐transaminases. Adv. Synth. Catal. 357, 1815–1821 (2015).
Börner, T. et al. Explaining operational instability of amine transaminases: substrate-induced inactivation mechanism and Influence of quaternary structure on enzyme–cofactor intermediate stability. ACS Catal. 7, 1259–1269 (2017).
Contente, M. L., Dall’Oglio, F., Tamborini, L., Molinari, F. & Paradisi, F. Highly efficient oxidation of amines to aldehydes with flow‐based biocatalysis. ChemCatChem 9, 3843–3848 (2017).
Quaglia, D. et al. His-tagged horse liver alcohol dehydrogenase: immobilisation and application in the bio-based enantioselective synthesis of (S)-arylpropanols. Process Biochem. 48, 810–818 (2013).
Contente, M. L. et al. Stereoselective reduction of aromatic ketones by a new ketoreductase from Pichia glucozyma. Appl. Microbiol. Biotechnol. 100, 193–201 (2016).
Satoha, Y., Tajima, K., Munekata, M., Keasling, J. D. & Lee, T. S. Engineering of l-tyrosine oxidation in Escherichia coli and microbial production of hydroxytyrosol. Metab. Eng. 14, 603–610 (2012).
Dubhashe, Y. R., Sawant, V. M. & Gaikar, V. G. Process intensification of continuous flow synthesis of tryptophol. Ind. Eng. Chem. Res. 57, 2787–2796 (2018).
Maślewski, P., Wyrzykowski, D., Witwicki, M. & Dołęga, A. Histaminol and its complexes with copper (ii)—studies in solid state solution. Eur. J. Inorg. Chem. 12, 1399–1408 (2018).
Bordiga, M., Travaglia, F., Locatelli, M., Arlorio, M. & Coisson, J. D. Histaminol: identification and HPLC analysis of a novel compound in wine. J. Agric. Food Chem. 58, 10202–10208 (2010).
Planchestainer, M. et al. Continuous flow biocatalysis: production and in-line purification of amines by immobilised transaminase from Halomonas elongata. Green. Chem. 19, 372–375 (2017).
Lichman, B. R. et al. One-pot triangular chemoenzymatic cascades for the synthesis of chiral alkaloids from dopamine. Green. Chem. 17, 852–855 (2015).
Orenes-Piñero, E., García-Carmona, F. & Sánchez-Ferrer, A. A new process for obtaining hydroxytirosol using transformed Escherichia coli whole cells with phenol hydroxylase from Geobacillus thermoglucosidasius. Food Chem. 139, 377–383 (2013).
Giacomini, D., Galletti, P., Quintavalla, A., Gucciardo, G. & Paradisi, F. Highly efficient asymmetric reduction of arylpropionic aldehydes by horse liver alcohol dehydrogenase through dynamic kinetic resolution. Chem. Commun. 39, 4038–4040 (2007).
Falus, P. et al. A continuous-flow cascade reactor system for subtilisin A-catalysed dynamic kinetic resolution of N-tert-butyloxycarbonylphenylalanine ethyl thioester with benzylamine. Adv. Synth. Catal. 358, 1608–1617 (2016).
Dall’Oglio, F. et al. Flow-based stereoselective reduction of ketones using an immobilised ketoreductase/glucose dehydrogenase mixed bed system. Catal. Commun. 93, 29–32 (2017).
De Maria, P. D. & Hollmann, F. On the (un)greenness of biocatalysis: some challenging figures and some promising options. Front. Microbiol 6, 1–5 (2015).
Rodríguez, C. et al. Steric vs. electronic effects in the Lactobacillis brevis ADH-catalysed bioreduction of ketones. Org. Biomol. Chem. 12, 673–681 (2014).
Cerioli, L., Planchestainer, M., Cassidy, J., Tessaro, D. & Paradisi, F. Characterization of a novel amine transaminase from Halomonas elongata. J. Mol. Cat. B 120, 141–150 (2015).
Acknowledgements
This work was supported by the Biotechnology and Biological Sciences Research Council [grant no. BB/P002536/1]; and the authors thank Resindion S.R.L. for donating the Sepabeads EC-EP/S.
Author information
Authors and Affiliations
Contributions
M.L.C. performed the experimental work and analysed the results. M.L.C. and F.P. conceived and designed the experiments. M.L.C. and F.P. co-wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Tables 1–4, Supplementary Equations 1 and 2, Supplementary Figures 1–3, Supplementary Note 1, Supplementary References, NMR Spectra
Rights and permissions
About this article
Cite this article
Contente, M.L., Paradisi, F. Self-sustaining closed-loop multienzyme-mediated conversion of amines into alcohols in continuous reactions. Nat Catal 1, 452–459 (2018). https://doi.org/10.1038/s41929-018-0082-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-018-0082-9
This article is cited by
-
Pickering emulsion droplets and solid microspheres acting synergistically for continuous-flow cascade reactions
Nature Catalysis (2024)
-
The joint effort of enzyme technology and flow chemistry to bring biocatalytic processes to the next level of sustainability, efficiency and productivity
Journal of Flow Chemistry (2024)
-
Perspectives on flow biocatalysis: the engine propelling enzymatic reactions
Journal of Flow Chemistry (2024)
-
Halomonas elongata: a microbial source of highly stable enzymes for applied biotechnology
Applied Microbiology and Biotechnology (2023)
-
Redox cascade reaction for kinetic resolution of racemic α-methylbenzylamine and biosynthesis of α-phenylethanol
Applied Microbiology and Biotechnology (2023)