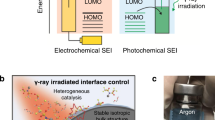
Abstract
The formation of solid electrolyte interphase on graphite anodes plays a key role in the efficiency of Li-ion batteries. However, to date, fundamental understanding of the formation of LiF as one of the main solid electrolyte interphase components in hexafluorophosphate-based electrolytes remains elusive. Here, we present experimental and theoretical evidence that LiF formation is an electrocatalytic process that is controlled by the electrochemical transformation of HF impurity to LiF and H2. Although the kinetics of HF dissociation and the concomitant production of LiF and H2 is dependent on the structure and nature of surface atoms, the underlying electrochemistry is the same. The morphology, and thus the role, of the LiF formed is strongly dependent on the nature of the substrate and HF inventory, leading to either complete or partial passivation of the interface. Our finding is of general importance and may lead to new opportunities for the improvement of existing, and design of new, Li-ion technologies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ross, P. N. & Lipkowski, J. Structure of Electrified Interfaces (VCH, New York, 1993).
Stamenkovic, V. R., Strmcnik, D., Lopes, P. P. & Markovic, N. M. Energy and fuels from electrochemical interfaces. Nat. Mater. 16, 57–69 (2017).
Wieckowski, A. Interfacial Electrochemistry (Marcel Dekker, New York, 1999).
Marković, N. & Ross, P. N. Surface science studies of model fuel cell electrocatalysts. Surf. Sci. Rep. 45, 117–229 (2002).
Nørskov, J. K., Bligaard, T., Rossmeisl, J. & Christensen, C. H. Towards the computational design of solid catalysts. Nat. Chem. 1, 37–46 (2009).
An, S. J. et al. The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon NY 105, 52–76 (2016).
Peled, E. & Menkin, S. Review—SEI: past, present and future. J. Electrochem. Soc. 164, A1703–A1719 (2017).
Verma, P., Maire, P. & Novák, P. A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries. Electrochim. Acta 55, 6332–6341 (2010).
Nie, M., Abraham, D. P., Chen, Y., Bose, A. & Lucht, B. L. Silicon solid electrolyte interphase (SEI) of lithium ion battery characterized by microscopy and spectroscopy. J. Phys. Chem. C 117, 13403–13412 (2013).
Owejan, J. E., Owejan, J. P., Decaluwe, S. C. & Dura, J. A. Solid electrolyte interphase in Li-ion batteries: evolving structures measured in situ by neutron reflectometry. Chem. Mater. 24, 2133–2140 (2012).
Soto, F. A., Ma, Y., Martinez De La Hoz, J. M., Seminario, J. M. & Balbuena, P. B. Formation and growth mechanisms of solid-electrolyte interphase layers in rechargeable batteries. Chem. Mater. 27, 7990–8000 (2015).
Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 104, 4303–4417 (2004).
Aurbach, D. Review of selected electrode—solution interactions which determine the performance of Li and Li ion batteries. J. Power Sources 89, 206–218 (2000).
Edström, K., Herstedt, M. & Abraham, D. P. A new look at the solid electrolyte interphase on graphite anodes in Li-ion batteries. J. Power Sources 153, 380–384 (2006).
Zhang, B. et al. Role of 1,3-propane sultone and vinylene carbonate in solid electrolyte interface formation and gas generation. J. Phys. Chem. C 119, 11337–11348 (2015).
Leroy, S., Martinez, H., Dedryvère, R., Lemordant, D. & Gonbeau, D. Influence of the lithium salt nature over the surface film formation on a graphite electrode in Li-ion batteries: an XPS study. Appl. Surf. Sci. 253, 4895–4905 (2007).
Leroy, S. et al. Surface film formation on a graphite electrode in Li-ion batteries: AFM and XPS study. Surf. Interface Anal. 37, 773–781 (2005).
Aurbach, D., Zinigrad, E., Cohen, Y. & Teller, H. A short review of failure mechanisms of lithium metal and lithiated graphite anodes in liquid electrolyte solutions. Solid State Ion. 148, 405–416 (2002).
Aurbach, D., Markovsky, B., Shechter, A., Ein-Eli, Y. & Cohen, H. A comparative study of synthetic graphite and Li electrodes in electrolyte solutions based on ethylene carbonate–dimethyl carbonate mixtures. J. Electrochem. Soc. 143, 3809–3820 (1996).
Lee, H., Cho, J.-J., Kim, J. & Kim, H.-J. Comparison of voltammetric responses over the cathodic region in LiPF6 and LiBETI with and without HF. J. Electrochem. Soc. 152, A1193–A1198 (2005).
Terborg, L. et al. Investigation of thermal aging and hydrolysis mechanisms in commercial lithium ion battery electrolyte. J. Power Sources 242, 832–837 (2013).
Kawamura, T., Okada, S. & Yamaki, J. Decomposition reaction of LiPF6-based electrolytes for lithium ion cells. J. Power Sources 156, 547–554 (2006).
Aurbach, D. et al. Recent studies on the correlation between surface chemistry, morphology, three-dimensional structures and performance of Li and Li-C intercalation anodes in several important electrolyte systems. J. Power Sources 68, 91–98 (1997).
Plakhotnyk, A. V., Ernst, L. & Schmutzler, R. Hydrolysis in the system LiPF6—propylene carbonate—dimethyl carbonate—H2O. J. Fluor. Chem. 126, 27–31 (2005).
Nakajima, T. & Groult, H. Fluorinated Materials for Energy Conversion (Elsevier, Oxford, 2005).
Lux, S. F. et al. The mechanism of HF formation in LiPF6 based organic carbonate electrolytes. Electrochem. Commun. 14, 47–50 (2012).
Aurbach, D. Electrode–solution interactions in Li-ion batteries: a short summary and new insights. J. Power Sources 119–121, 497–503 (2003).
Zhang, Z. & Zhang, S. S. Rechargeable Batteries: Materials, Technologies and New Trends (Springer, New York, 2015).
Wang, R., Li, X., Wang, Z. & Guo, H. Manganese dissolution from LiMn2O4 cathodes at elevated temperature: methylene methanedisulfonate as electrolyte additive. J. Solid State Electrochem. 20, 19–28 (2016).
Pieczonka, N. P. W. et al. Understanding transition-metal dissolution behavior in LiNi0.5Mn1.5O4 high-voltage spinel for lithium ion batteries. J. Phys. Chem. C 117, 15947–15957 (2013).
Kang, S. J., Yu, S., Lee, C., Yang, D. & Lee, H. Effects of electrolyte-volume-to-electrode-area ratio on redox behaviors of graphite anodes for lithium-ion batteries. Electrochim. Acta 141, 367–373 (2014).
Strmcnik, D. et al. When small is big: the role of impurities in electrocatalysis. Top. Catal. 58, 1174–1180 (2015).
Marković, N., Gasteiger, H., Grgur, B. & Ross, P. Oxygen reduction reaction on Pt(111): effects of bromide. J. Electroanal. Chem. 467, 157–163 (1999).
Strmcnik, D. S. et al. Unique activity of platinum adislands in the CO electrooxidation reaction. J. Am. Chem. Soc. 130, 15332–15339 (2008).
Subbaraman, R. et al. Origin of anomalous activities for electrocatalysts in alkaline electrolytes. J. Phys. Chem. C 116, 22231–22237 (2012).
Vetter, K. J. Electrochemical Kinetics: Theoretical and Experimental Aspects (Academic, New York, 1967).
Thiel, P. A., Madey, T. E. & Sloan, A. P. The interaction of water with solid surfaces: fundamental aspects. Surf. Sci. Rep. 7, 211–385 (1987).
Henderson, M. A. The interaction of water with solid surfaces: fundamental aspects revisited. Surf. Sci. Rep. 46, 1–308 (2002).
Trasatti, S. Work function, electronegativity, and electrochemical behaviour of metals: III. Electrolytic hydrogen evolution in acid solutions. Electroanal. Chem. Interfac. Electrochem. 39, 163–184 (1972).
Greeley, J., Jaramillo, T. F., Bonde, J., Chorkendorff, I. & Nørskov, J. K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 5, 909–913 (2006).
Strmcnik, D., Lopes, P. P., Genorio, B., Stamenkovic, V. R. & Markovic, N. M. Design principles for hydrogen evolution reaction catalyst materials. Nano Energy 29, 29–36 (2016).
Staszak-Jirkovský, J. et al. Water as a promoter and catalyst for dioxygen electrochemistry in aqueous and organic media. ACS Catal. 5, 6600–6607 (2015).
Maibach, J., Lindgren, F., Eriksson, H., Edström, K. & Hahlin, M. Electric potential gradient at the buried interface between lithium-ion battery electrodes and the SEI observed using photoelectron spectroscopy. J. Phys. Chem. Lett. 7, 1775–1780 (2016).
Matsuoka, O. et al. Ultra-thin passivating film induced by vinylene carbonate on highly oriented pyrolytic graphite negative electrode in lithium-ion cell. J. Power Sources 108, 128–138 (2002).
Alliata, D., Kötz, R., Novák, P. & Siegenthaler, H. Electrochemical SPM investigation of the solid electrolyte interphase film formed on HOPG electrodes. Electrochem. Commun. 2, 436–440 (2000).
Jeong, S.-K., Inaba, M., Abe, T. & Ogumi, Z. Surface film formation on graphite negative electrode in lithium-ion batteries: AFM study in an ethylene carbonate-based solution. J. Electrochem. Soc. 148, A989–A993 (2001).
Domi, Y. et al. Electrochemical AFM observation of the HOPG edge plane in ethylene carbonate-based electrolytes containing film-forming additives. J. Electrochem. Soc. 159, A1292–A1297 (2012).
Domi, Y. et al. In situ AFM study of surface film formation on the edge plane of HOPG for lithium-ion batteries. J. Phys. Chem. C 115, 25484–25489 (2011).
Peled, E. et al. Composition, depth profiles and lateral distribution of materials in the SEI built on HOPG-TOF SIMS and XPS studies. J. Power Sources 97–98, 52–57 (2001).
Metzger, M., Strehle, B., Solchenbach, S. & Gasteiger, H. A. Origin of H2 evolution in LIBs: H2O reduction vs. electrolyte oxidation. J. Electrochem. Soc. 163, A798–A809 (2016).
Mortensen, J. J., Hansen, L. B. & Jacobsen, K. W. Real-space grid implementation of the projector augmented wave method. Phys. Rev. B Condens. Matter Mater. Phys. 71, 1–11 (2005).
Enkovaara, J. et al. Electronic structure calculations with GPAW: a real-space implementation of the projector augmented-wave method. J. Phys. Condens. Matter 22, 253202 (2010).
Hjorth Larsen, A. et al. The atomic simulation environment—a Python library for working with atoms. J. Phys. Condens. Matter 29, 273002 (2017).
Hammer, B., Hansen, L. & Nørskov, J. Improved adsorption energetics within density-functional theory using revised Perdew–Burke–Ernzerhof functionals. Phys. Rev. B 59, 7413–7421 (1999).
Hansen, M. H. & Rossmeisl, J. pH in grand canonical statistics of an electrochemical interface. J. Phys. Chem. C 120, 29135–29143 (2016).
Acknowledgements
This research was sponsored by BMW Technology Corporation. The research was conducted at Argonne National Laboratory—a US Department of Energy Office of Science laboratory operated by UChicago Argonne under contract number DE-AC02-06CH11357 and in part at the Technische Universität, München. We acknowledge support from the Office of Science, Office of Basic Energy Sciences and Materials Sciences and Engineering Division. We also thank C. Thompson and H. You for help with the AFM and crystal truncation rod measurements.
Author information
Authors and Affiliations
Contributions
D.S., J.G.C., H.A.G. and N.M.M. conceived and designed the experiments. D.S., J.G.C., D.H., M.Z., P.M., P.P.L. and B.G. performed the experiments. I.E.C., T.Ø. and J.R. performed the calculations. D.S., J.G.C., I.E.C., F.M., B.K.A., J.R., H.A.G., V.R.S. and N.M.M. discussed the results and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Figures 1–16, Supplementary Tables 1–3, Supplementary References.
Rights and permissions
About this article
Cite this article
Strmcnik, D., Castelli, I.E., Connell, J.G. et al. Electrocatalytic transformation of HF impurity to H2 and LiF in lithium-ion batteries. Nat Catal 1, 255–262 (2018). https://doi.org/10.1038/s41929-018-0047-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-018-0047-z
This article is cited by
-
Near ambient N2 fixation on solid electrodes versus enzymes and homogeneous catalysts
Nature Reviews Chemistry (2023)
-
Gas induced formation of inactive Li in rechargeable lithium metal batteries
Nature Communications (2023)
-
Electrochemical Scanning Tunneling Microscopy as a Tool for the Detection of Active Electrocatalytic Sites
Topics in Catalysis (2023)
-
Revealing solid electrolyte interphase formation through interface-sensitive Operando X-ray absorption spectroscopy
Nature Communications (2022)
-
Dynamic observation of dendrite growth on lithium metal anode during battery charging/discharging cycles
npj Computational Materials (2022)