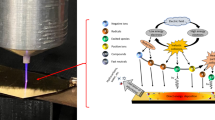
Abstract
Correlations between the energies of elementary steps limit the rates of thermally catalysed reactions at surfaces. Here, we show how these limitations can be circumvented in ammonia synthesis by coupling catalysts to a non-thermal plasma. We postulate that plasma-induced vibrational excitations in N2 decrease dissociation barriers without influencing subsequent reaction steps. We develop a density-functional-theory-based microkinetic model to incorporate this effect, and parameterize the model using N2 vibrational excitations observed in a dielectric-barrier-discharge plasma. We predict plasma enhancement to be particularly great on metals that bind nitrogen too weakly to be active thermally. Ammonia synthesis rates observed in a dielectric-barrier-discharge plasma reactor are consistent with predicted enhancements and predicted changes in the optimal metal catalyst. The results provide guidance for optimizing catalysts for application with plasmas.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Abild-Pedersen, F. et al. Scaling properties of adsorption energies for hydrogen-containing molecules on transition-metal surfaces. Phys. Rev. Lett. 99, 016105 (2007).
Evans, M. G. & Polanyi, M. Inertia and driving force of chemical reactions. Trans. Faraday Soc. 34, 11–24 (1938).
Bligaard, T. et al. The Brønsted–Evans–Polanyi relation and the volcano curve in heterogeneous catalysis. J. Catal. 224, 206–217 (2004).
Vojvodic, A. & Norskov, J. K. New design paradigm for heterogeneous catalysts. Natl Sci. Rev. 2, 140–149 (2015).
Greeley, J. Theoretical heterogeneous catalysis: scaling relationships and computational catalyst design. Annu. Rev. Chem. Biomol. Eng. 7, 605–635 (2016).
Medford, A. J. et al. From the Sabatier principle to a predictive theory of transition-metal heterogeneous catalysis. J. Catal. 328, 36–42 (2015).
Logadottir, A. et al. The Brønsted–Evans–Polanyi relation and the volcano plot for ammonia synthesis over transition metal catalysts. J. Catal. 197, 229–231 (2001).
Vojvodic, A. et al. Exploring the limits: a low-pressure, low-temperature Haber–Bosch process. Chem. Phys. Lett. 598, 108–112 (2014).
Nørskov, J., Chen, J., Miranda, R., Fitzsimmons, T. & Stack, R. Sustainable Ammonia Synthesis—Exploring the Scientific Challenges Associated with Discovering Alternative, Sustainable Processes for Ammonia Production (US Department of Energy Office of Science, 2016).
Bai, M., Zhang, Z., Bai, X., Bai, M. & Ning, W. Plasma synthesis of ammonia with a microgap dielectric barrier discharge at ambient pressure. IEEE Trans. Plasma Sci. 31, 1285–1291 (2003).
Mizushima, T., Matsumoto, K., Sugoh, J., Ohkita, H. & Kakuta, N. Tubular membrane-like catalyst for reactor with dielectric-barrier-discharge plasma and its performance in ammonia synthesis. Appl. Catal. A 265, 53–59 (2004).
Mizushima, T., Matsumoto, K., Ohkita, H. & Kakuta, N. Catalytic effects of metal-loaded membrane-like alumina tubes on ammonia synthesis in atmospheric pressure plasma by dielectric barrier discharge. Plasma Chem. Plasma Process. 27, 1–11 (2006).
Gómez-Ramírez, A., Cotrino, J., Lambert, R. M. & González-Elipe, A. R. Efficient synthesis of ammonia from N2 and H2 alone in a ferroelectric packed-bed DBD reactor. Plasma Sources Sci. Technol. 24, 065011 (2015).
Kim, H.-H., Teramoto, Y., Ogata, A., Takagi, H. & Nanba, T. Atmospheric-pressure nonthermal plasma synthesis of ammonia over ruthenium catalysts. Plasma Process. Polym. 14, 1600157 (2016).
Peng, P. et al. Atmospheric pressure ammonia synthesis using non-thermal plasma assisted catalysis. Plasma Chem. Plasma Process. 36, 1201–1210 (2016).
Xie, D. et al. Ammonia synthesis and by-product formation from H2O, H2 and N2 by dielectric barrier discharge combined with an Ru/Al2O3 catalyst. RSC Adv. 6, 105338–105346 (2016).
Hong, J. et al. Plasma catalytic synthesis of ammonia using functionalized-carbon coatings in an atmospheric-pressure non-equilibrium discharge. Plasma Chem. Plasma Process. 36, 917–940 (2016).
Akay, G. & Zhang, K. Process intensification in ammonia synthesis using novel coassembled supported microporous catalysts promoted by nonthermal plasma. Ind. Eng. Chem. Res. 56, 457–468 (2017).
Iwamoto, M., Akiyama, M., Aihara, K. & Deguchi, T. Ammonia synthesis on wool-like Au, Pt, Pd, Ag, or Cu electrode catalysts in nonthermal atmospheric-pressure plasma of N2 and H2. ACS Catal. 7, 6924–6929 (2017).
Akay, G. Ammonia production by integrated intensified processes. US Patent 20130309161A1 (2011).
Fridman, A. Plasma Chemistry. (Cambridge Univ. Press, New York, NY, 2008).
Rettner, C. T. & Stein, H. Effect of vibrational energy on the dissociative chemisorption of N2 on Fe(111). J. Chem. Phys. 87, 770–771 (1987).
Holmblad, P. M., Wambach, J. & Chorkendorff, I. Molecular beam study of dissociative sticking of methane on Ni(100). J. Chem. Phys. 102, 8255–8263 (1995).
Romm, L., Katz, G., Kosloff, R. & Asscher, M. Dissociative chemisorption of N2 on Ru(001) enhanced by vibrational and kinetic energy: molecular beam experiments and quantum mechanical calculations. J. Phys. Chem. B 101, 2213–2217 (1997).
Murphy, M. J., Skelly, J. F., Hodgson, A. & Hammer, B. Inverted vibrational distributions from N2 recombination at Ru(001): evidence for a metastable molecular chemisorption well. J. Chem. Phys. 110, 6954–6962 (1999).
Luntz, A. C. A simple model for associative desorption and dissociative chemisorption. J. Chem. Phys. 113, 6901–6905 (2000).
Diekhöner, L. et al. N2 dissociative adsorption on Ru(0001): the role of energy loss. J. Chem. Phys. 115, 9028–9035 (2001).
Smith, R. R. Preference for vibrational over translational energy in a gas-surface reaction. Science 304, 992–995 (2004).
Ertl, G. in Elementary Steps in Ammonia Synthesis 109–132 (Springer, Boston, MA, 1991).
Honkala, K. Ammonia synthesis from first-principles calculations. Science 307, 555–558 (2005).
Wang, S. et al. Universal transition state scaling relations for (de)hydrogenation over transition metals. Phys. Chem. Chem. Phys. 13, 20760 (2011).
Hummelshøj, J. S., Abild-Pedersen, F., Studt, F., Bligaard, T. & Nørskov, J. K. CatApp: a web application for surface chemistry and heterogeneous catalysis. Angew. Chem. Int. Ed. 124, 278–280 (2011).
Falsig, H. et al. On the structure sensitivity of direct no decomposition over low-index transition metal facets. Top. Catal. 57, 80–88 (2013).
Grabow, L. C. in Computational Catalysis 1–58 (Royal Society of Chemistry, Cambridge, 2013).
Hansen, F. Y., Henriksen, N. E., Billing, G. D. & Guldberg, A. Catalytic synthesis of ammonia using vibrationally excited nitrogen molecules: theoretical calculation of equilibrium and rate constants. Surf. Sci. 264, 225–234 (1992).
Polanyi, J. C. Concepts in reaction dynamics. Acc. Chem. Res. 5, 161–168 (1972).
Díaz, C. & Olsen, R. A. A note on the vibrational efficacy in molecule-surface reactions. J. Chem. Phys. 130, 094706 (2009).
Cacciatore, M., Capitelli, M., De Benedictis, S., Dilonardo, M. & Gorse, C. in Nonequilibrium Vibrational Kinetics 5–46 (Springer, Berlin and Heidelberg, 1986).
Laux, C. O., Spence, T. G., Kruger, C. H. & Zare, R. N. Optical diagnostics of atmospheric pressure air plasmas. Plasma Sources Sci. Technol. 12, 125–138 (2003).
Treanor, C. E., Rich, J. W. & Rehm, R. G. Vibrational relaxation of anharmonic oscillators with exchange-dominated collisions. J. Chem. Phys. 48, 1798–1807 (1968).
Gordiets, B. F. & Zhdanok, S. in Nonequilibrium Vibrational Kinetics 47–84 (Springer, Berlin and Heidelberg, 1986).
Kim, J., Go, D. B. & Hicks, J. C. Synergistic effects of plasma–catalyst interactions for CH4 activation. Phys. Chem. Chem. Phys. 19, 13010–13021 (2017).
Kim, J., Abbott, M. S., Go, D. B. & Hicks, J. C. Enhancing C–H bond activation of methane via temperature-controlled, catalyst-plasma interactions. ACS Energy Lett. 1, 94–99 (2016).
Neyts, E. C., Ostrikov, K. K., Sunkara, M. K. & Bogaerts, A. Plasma catalysis: synergistic effects at the nanoscale. Chem. Rev. 115, 13408–13446 (2015).
Snoeckx, R. & Bogaerts, A. Plasma technology—a novel solution for CO2 conversion? Chem. Soc. Rev. 46, 5805–5863 (2017).
Wang, W., Patil, B., Heijkers, S., Hessel, V. & Bogaerts, A. Nitrogen fixation by gliding arc plasma: better insight by chemical kinetics modelling. ChemSusChem 10, 2145–2157 (2017).
Hammer, B., Hansen, L. B. & Nørskov, J. K. Improved adsorption energetics within density-functional theory using revised Perdew–Burke–Ernzerhof functionals. Phys. Rev. B 59, 7413–7421 (1999).
Johnson III, R. D. NIST 101. Computational Chemistry Comparison and Benchmark Database (National Institute of Standards and Technology, 1999).
Lausche, A. C. et al. On the effect of coverage-dependent adsorbate–adsorbate interactions for CO methanation on transition metal surfaces. J. Catal. 307, 275–282 (2013).
Frey, K., Schmidt, D. J., Wolverton, C. & Schneider, W. F. Implications of coverage-dependent O adsorption for catalytic NO oxidation on the late transition metals. Catal. Sci. Technol. 4, 4356–4365 (2014).
Heijkers, S. et al. CO2 conversion in a microwave plasma reactor in the presence of N2: elucidating the role of vibrational levels. J. Phys. Chem. C 119, 12815–12828 (2015).
Li, S., Ma, C., Zhang, Q., Ren, C. & Lu, W. Ion nitriding of pure iron using high-density plasma beam generated by a tubular plasma source. Surf. Coat. Technol. 309, 47–53 (2017).
Staack, D., Farouk, B., Gutsol, A. & Fridman, A. Characterization of a DC atmospheric pressure normal glow discharge. Plasma Sources Sci. Technol. 14, 700–711 (2005).
Staack, D., Farouk, B., Gutsol, A. F. & Fridman, A. A. Spectroscopic studies and rotational and vibrational temperature measurements of atmospheric pressure normal glow plasma discharges in air. Plasma Sources Sci. Technol. 15, 818–827 (2006).
Laux, C. in Physico-Chemical Modeling of High Enthalpy and Plasma Flows 1–55 (von Karman Institute Lecture Series 2002-07, Rhode-Saint-Genèse, 2002).
Hummelt, J. S., Shapiro, M. A. & Temkin, R. J. Spectroscopic temperature measurements of air breakdown plasma using a 110 GHz megawatt gyrotron beam. Phys. Plasmas 19, 123509 (2012).
Acknowledgements
This work was supported by the US Department of Energy, Office of Science, Basic Energy Sciences, Sustainable Ammonia Synthesis Program, under award number DE-SC-0016543. W.F.S. acknowledges additional support under award number DE-FG02-06ER15839. Computational resources were provided by the Notre Dame Center for Research Computing. We thank the Notre Dame Energy Materials Characterization Facility and Notre Dame Integrated Imaging Facility for use of the X-ray diffractometer and transmission electron microscope, respectively. P.M. acknowledges support through the Eilers Graduate Fellowship for Energy Related Research.
Author information
Authors and Affiliations
Contributions
P.M. developed the microkinetic model. P.B. and J.K. performed the ammonia synthesis rate experiments. F.A.H. and P.R. performed the plasma characterization. P.M., P.B., F.A.H., D.B.G, J.C.H. and W.F.S. co-wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Figures 1–11, Supplementary Table 1 and Supplementary References.
Supplementary Data
Python source code.
Rights and permissions
About this article
Cite this article
Mehta, P., Barboun, P., Herrera, F.A. et al. Overcoming ammonia synthesis scaling relations with plasma-enabled catalysis. Nat Catal 1, 269–275 (2018). https://doi.org/10.1038/s41929-018-0045-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-018-0045-1
This article is cited by
-
Bias-free solar NH3 production by perovskite-based photocathode coupled to valorization of glycerol
Nature Catalysis (2024)
-
Enhancements of electric field and afterglow of non-equilibrium plasma by Pb(ZrxTi1−x)O3 ferroelectric electrode
Nature Communications (2024)
-
Achieving volatile potassium promoted ammonia synthesis via mechanochemistry
Nature Communications (2023)
-
Programmable catalysis by support polarization: elucidating and breaking scaling relations
Nature Communications (2023)
-
Current state and future prospects of liquid metal catalysis
Nature Catalysis (2023)