Abstract
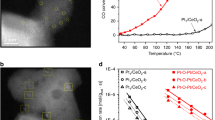
Single-atom catalysts have attracted great attention in recent years due to their high efficiencies and cost savings. However, there is debate concerning the nature of the active site, interaction with the support, and mechanism by which single-atom catalysts operate. Here, using a combined surface science and theory approach, we designed a model system in which we unambiguously show that individual Pt atoms on a well-defined Cu2O film are able to perform CO oxidation at low temperatures. Isotopic labelling studies reveal that oxygen is supplied by the support. Density functional theory rationalizes the reaction mechanism and confirms X-ray photoelectron spectroscopy measurements of the neutral charge state of Pt. Scanning tunnelling microscopy enables visualization of the active site as the reaction progresses, and infrared measurements of the CO stretch frequency are consistent with atomically dispersed Pt atoms. These results serve as a benchmark for characterizing, understanding and designing other single-atom catalysts.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Thomas, J. M. The concept, reality and utility of single-site heterogeneous catalysts (SSHCs). Phys. Chem. Chem. Phys. 16, 7647–7661 (2014).
Liu, J. Catalysis by supported metal single atoms. ACS Catal. 7, 34–59 (2017).
Flytzani-Stephanopoulos, M. & Gates, B. C. Atomically dispersed supported metal catalysts. Annu. Rev. Chem. Biomol. Eng. 3, 545–574 (2012).
Nie, L. et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science 358, 1419–1423 (2017).
Yang, M. et al. Catalytically active Au–O(OH)x-species stabilized by alkali ions on zeolites and mesoporous oxides. Science 346, 1498–1501 (2014).
Yang, M. et al A common single-site Pt(II)–O(OH)x—species stabilized by sodium on ‘active’ and ‘inert’ supports catalyzes the water–gas shift reaction. J. Am. Chem. Soc. 137, 3470–3473 (2015).
Zhai, Y. et al. Alkali-stabilized Pt–OHx species catalyze low-temperature water–gas shift reactions. Science 329, 1633–1636 (2010).
Hutchings, G. J. et al. Role of gold cations in the oxidation of carbon monoxide catalyzed by iron oxide-supported gold. J. Catal. 242, 71–81 (2006).
Liu, S. et al. Stabilizing single-atom and small-domain platinum via combining organometallic chemisorption and atomic layer deposition. Organometallics 36, 818–828 (2017).
Ding, K. et al. Identification of active sites in CO oxidation and water–gas shift over supported Pt catalysts. Science 350, 189–192 (2015).
Herzing, A. A., Kiely, C. J., Carley, A. F., Landon, P. & Hutchings, G. J. Identification of active gold nanoclusters on iron oxide supports for CO oxidation. Science 321, 1331–1335 (2008).
Ulrich, S. et al. Evidence for a size-selective adsorption mechanism on oxide surfaces: Pd and Au atoms on SiO2/Mo(112). ChemPhysChem 9, 1367–1370 (2008).
Bliem, R. et al. Cluster nucleation and growth from a highly supersaturated adatom phase: silver on magnetite. ACS Nano 8, 7531–7537 (2014).
Giordano, L. et al. Charging of metal adatoms on ultrathin oxide films: Au and Pd on FeO/Pt(111). Phys. Rev. Lett. 101, 26102 (2008).
Novotný, Z. et al. Ordered array of single adatoms with remarkable thermal stability: Au/Fe3O4(001). Phys. Rev. Lett. 108, 216103 (2012).
Skomski, D., Tempas, C. D., Smith, K. A. & Tait, S. L. Redox-active on-surface assembly of metal−organic chains with single-site Pt(II). J. Am. Chem. Soc. 136, 9862–9865 (2014).
Skomski, D. et al. Two- and three-electron oxidation of single-site vanadium centers at surfaces by ligand design. J. Am. Chem. Soc. 137, 7898–7902 (2015).
Rim, K. T. et al. Charging and chemical reactivity of gold nanoparticles and adatoms on the (111) surface of single-crystal magnetite: a scanning tunneling microscopy/spectroscopy study. J. Phys. Chem. C 113, 10198–10205 (2009).
Yang, B., Lin, X., Gao, H.-J., Nilius, N. & Freund, H.-J. CO adsorption on thin MgO films and single Au adatoms: a scanning tunneling microscopy study. J. Phys. Chem. C 114, 8997–9001 (2010).
Parkinson, G. S. et al. Carbon monoxide-induced adatom sintering in a Pd–Fe3O4 model catalyst. Nat. Mater. 12, 724–728 (2013).
Bliem, R. et al. Dual role of CO in the stability of subnano Pt clusters at the Fe3O4(001) surface. Proc. Natl Acad. Sci. USA 113, 8921–8926 (2016).
Zhou, X. et al. Stable Pt single atoms and nanoclusters on ultrathin CuO film and their performances in CO oxidation. J. Phys. Chem. C 120, 1709–1715 (2016).
Bliem, R. et al. An atomic-scale view of CO and H2 oxidation on a Pt/Fe3O4 model catalyst. Angew. Chem. Int. Ed. Engl. 54, 13999–14002 (2015).
Therrien, A. J. et al. Structurally accurate model for the ‘29’-structure of CuxO/Cu(111): a DFT and STM study. J. Phys. Chem. C 120, 10879–10886 (2016).
Hensley, A. J. R. et al. CO adsorption on the ‘29’ CuxO/Cu(111) surface: an integrated DFT, STM and TPD study. J. Phys. Chem. C 120, 25387–25394 (2016).
Mukerji, R. J., Bolina, A. S. & Brown, W. A. A RAIRS and TPD investigation of the adsorption of CO on Pt{211}. Surf. Sci. 527, 198–208 (2003).
Hayden, B. E. & Bradshaw, A. M. The adsorption of CO on Pt(111) studied by infrared-reflection-adsorption spectroscopy. Surf. Sci. 125, 787–802 (1983).
Orita, H. & Inada, Y. DFT investigation of CO adsorption on Pt(211) and Pt (311) surfaces from low to high coverage. J. Phys. Chem. B 109, 22469–22475 (2005).
Lundwall, M. J., Mcclure, S. M. & Goodman, D. W. Probing terrace and step sites on Pt nanoparticles using CO and ethylene. J. Phys. Chem. C 114, 7904–7912 (2010).
Hoffman, A. S., Fang, C.-Y. & Gates, B. C. Homogeneity of surface sites in supported single-site metal catalysts: assessment with band widths of metal carbonyl infrared spectra. J. Phys. Chem. Lett. 7, 3854–3860 (2016).
Liu, L. et al. Generation of subnanometric platinum with high stability during transformation of a 2D zeolite into 3D. Nat. Mater. 16, 132–138 (2017).
Matsubu, J. C., Yang, V. N. & Christopher, P. Isolated metal active site concentration and stability control catalytic CO2 reduction selectivity. J. Am. Chem. Soc. 137, 3076–3084 (2015).
Lee, H. & Ho, W. Structural determination by single-molecule vibrational spectroscopy and microscopy: contrast between copper and iron carbonyls. Phys. Rev. B 61, R16347–R16350 (2000).
Chen, S. et al. Probing surface structures of CeO2, TiO2, and Cu2O nanocrystals with CO and CO2 chemisorption. J. Phys. Chem. C 120, 21472–21485 (2016).
Baber, A. E. et al. Stabilization of catalytically active Cu+ surface sites on titanium-copper mixed-oxide films. Angew. Chem. Int. Ed. Engl. 126, 5440–5444 (2014).
Baber, A. E. et al. In situ imaging of Cu2O under reducing conditions: formation of metallic fronts by mass transfer. J. Am. Chem. Soc. 135, 16781–16784 (2013).
Gerrard, A. L. & Weaver, J. F. Kinetics of CO oxidation on high-concentration phases of atomic oxygen on Pt(111). J. Chem. Phys. 123, 224703 (2005).
Heiz, U., Sanchez, A., Abbet, S. & Schneider, W.-D. Catalytic oxidation of carbon monoxide on monodispersed platinum clusters: each atom counts. J. Am. Chem. Soc. 121, 3214–3217 (1999).
Xu, J. & Yates, J. T. Catalytic oxidation of CO on Pt(335): a study of the active site. J. Chem. Phys. 99, 725–732 (1993).
Campbell, C. T., Ertl, G., Kuipers, H. & Segner, J. A molecular beam investigation of the interactions of CO with a Pt(111) surface. Surf. Sci. 107, 207–219 (1981).
Liu, J. et al. Tackling CO poisoning with single-atom alloy catalysts. J. Am. Chem. Soc. 138, 6396–6399 (2016).
National Archives and Records Administration Greenhouse gas emissions and fuel efficiency standards for medium- and heavy-duty engines and vehicles—phase 2. Fed. Regist. 81, 73478–74274 (2016)..
Doornkamp, C. & Ponec, V. The universal character of the Mars and Van Krevelen mechanism. J. Mol. Catal. A Chem. 162, 19–32 (2000).
Redhead, P. A. Thermal desorption of gases. Vacuum 12, 203–211 (1962).
King, D. A. Thermal desorption from metal surfaces: a review. Surf. Sci. 47, 384–402 (1975).
Koslowski, B., Dietrich, C., Tschetschetkin, A. & Ziemann, P. Evaluation of scanning tunneling spectroscopy data: approaching a quantitative determination of the electronic density of states. Phys. Rev. B 75, 35421 (2007).
Lang, N. D. Spectroscopy of single atoms in the scanning tunneling microscope. Phys. Rev. B 34, 5947–5950 (1986).
Giordano, L. & Pacchioni, G. Oxide film at the nanoscale: new structures, new functions, and new materials. Acc. Chem. Res. 44, 1244–1252 (2011).
Jones, J. et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 353, 150–154 (2016).
Lang, R. et al. Hydroformylation of olefins by a rhodium single-atom catalyst with activity comparable to RhCl(PPh3)3. Angew. Chem. Int. Ed. Engl. 55, 16054–16058 (2016).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Pack, J. D. & Monkhorst, H. J. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Deringer, V. L., Tchougréeff, A. L. & Dronskowski, R. Crystal orbital Hamilton population (COHP) analysis as projected from plane-wave basis sets. J. Phys. Chem. A 115, 5461–5466 (2011).
Maintz, S., Deringer, V. L., Tchougréeff, A. L. & Dronskowski, R. Analytic projection from plane-wave and PAW wavefunctions and application to chemical-bonding analysis in solids. J. Comput. Chem. 34, 2557–2567 (2013).
Henkelman, G. & Jónsson, H. A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives. J. Chem. Phys. 111, 7010–7022 (1999).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. Climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Trygubenko, S. A. & Wales, D. J. A doubly nudged elastic band method for finding transition states. J. Chem. Phys. 120, 2082–2094 (2004).
Hammer, B., Hansen, L. B. & Nørskov, J. K. Improved adsorption energetics within density-functional theory using revised Perdew–Burke–Ernzerhof functionals. Phys. Rev. B 59, 7413–7421 (1999).
Dion, M., Rydberg, H., Schröder, E., Langreth, D. C. & Lundqvist, B. I. Van der Waals density functional for general geometries. Phys. Rev. Lett. 92, 246401 (2004).
Dudarev, S. L., Savrasov, S. Y., Humphreys, C. J. & Sutton, A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA+U study. Phys. Rev. B 57, 1505–1509 (1998).
Acknowledgements
The surface science work at Tufts was supported by the Department of Energy Basic Energy Sciences programme under grant number DE-FG02-05ER15730. M.D.M. thanks Tufts Chemistry for an Illumina Fellowship. Financial support at Washington State University was provided by the National Science Foundation Early-concept Grants for Exploratory Research programme under contract number CBET-1552320 and the CAREER programme under contract number CBET-1653561. Our thanks also go to the donors of the American Chemical Society Petroleum Research Fund. A portion of the computer time for the computational work was performed at the Environmental Molecular Sciences Laboratory—a national scientific user facility sponsored by the Department of Energy’s Office of Biological and Environmental Research and located at the Pacific Northwest National Laboratory. The Pacific Northwest National Laboratory is a multi-programme national laboratory operated for the US Department of Energy by Battelle.
Author information
Authors and Affiliations
Contributions
A.J.T. carried out the sample preparation as well as the STM, STS, TPD, XPS and RAIRS experiments, and assisted with writing the manuscript. A.J.R.H. carried out the DFT calculations and assisted with writing the manuscript. M.D.M. assisted with the TPD and STM experiments. R.Z. assisted with the DFT calculations. F.R.L. assisted with the STM imaging and STS experiments. B.C. and A.C.S. assisted with the STM imaging and XPS experiments. J.-S.M. oversaw and guided the DFT calculations and assisted with writing the manuscript. E.C.H.S. conceived the project, directed the study and assisted with writing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Discussion, Supplementary Figures 1–7, Supplementary Table 1 and Supplementary References
Rights and permissions
About this article
Cite this article
Therrien, A.J., Hensley, A.J.R., Marcinkowski, M.D. et al. An atomic-scale view of single-site Pt catalysis for low-temperature CO oxidation. Nat Catal 1, 192–198 (2018). https://doi.org/10.1038/s41929-018-0028-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-018-0028-2
This article is cited by
-
Interplay between geometric and electronic structures of Pt entities over TiO2 for CO oxidation
Science China Chemistry (2024)
-
Sb2S3-templated synthesis of sulfur-doped Sb-N-C with hierarchical architecture and high metal loading for H2O2 electrosynthesis
Nature Communications (2023)
-
Synergistic roles of platinum nanoparticles and sodium ions within beta zeolites in N-alkylation of amines with aromatic alcohols
Science China Chemistry (2023)
-
The concept of active site in heterogeneous catalysis
Nature Reviews Chemistry (2022)
-
Abrading bulk metal into single atoms
Nature Nanotechnology (2022)