Abstract
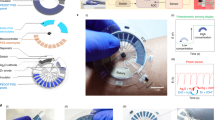
Flexible chemical sensors could be used to create wearable devices that continuously monitor a variety of health conditions through the analysis of a person’s sweat. Current wearable pH sensors for sweat monitoring have a limited pH detection sensitivity at room temperature of about 59 millivolts per pH unit, which is determined by the Nernst equation. Here we show that a sensitive pH sensor can be created using a flexible charge-coupled device (CCD) and integrated with a temperature sensor. Our CCD-based pH sensor can, through accumulation cycles of electron charge transfer, achieve a sensitivity of around 240 millivolts per pH unit, which is roughly four times larger than the Nernst theoretical limit. Furthermore, the integrated flexible temperature sensor can be simultaneously used to compensate for the temperature dependence of the pH detection and to monitor skin temperature. As a proof of concept, we demonstrate that our CCD-based chemical sensor can be used to monitor the sweat pH and skin temperature of a person in real time.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the finding of this study are available from the corresponding author upon reasonable request.
References
Cao, Q. et al. Medium-scale carbon nanotube thin-film integrated circuits on flexible plastic substrates. Nature 454, 495–500 (2008).
Kim, D. H. et al. Materials for multifunctional balloon catheters with capabilities in cardiac electrophysiological mapping and ablation therapy. Nat. Mater. 10, 316–323 (2011).
Someya, T. et al. Conformable, flexible, large-area networks of pressure and thermal sensors with organic transistor active matrixes. Proc. Natl Acad. Sci. USA 102, 12321–12325 (2005).
Wang, S. et al. Skin electronics from scalable fabrication of an intrinsically stretchable transistor array. Nature 555, 83–88 (2018).
Takei, K. et al. Nanowire active-matrix circuitry for low-voltage macroscale artificial skin. Nat. Mater. 9, 821–826 (2010).
Chen, H., Cao, Y., Zhang, J. & Zhou, C. Large-scale complementary macroelectronics using hybrid integration of carbon nanotubes and IGZO thin-film transistors. Nat. Commun. 5, 4097 (2014).
Honda, W. et al. High-performance, mechanically flexible, and vertically integrated 3D carbon nanotube and InGaZnO complementary circuits with a temperature sensor. Adv. Mater. 27, 4674–4680 (2015).
Choi, S., Lee, H., Ghaffari, R., Hyeon, T. & Kim, D. H. Recent advances in flexible and stretchable bio-electronic devices integrated with nanomaterials. Adv. Mater. 28, 4203–4218 (2016).
Chortos, A., Liu, J. & Bao, Z. Pursuing prosthetic electronic skin. Nat. Mater. 15, 937–950 (2016).
Gao, W. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514 (2016).
Kim, D. H. et al. Epidermal electronics. Science 333, 838–843 (2011).
Lee, H. et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotech. 11, 566–572 (2016).
Lee, H. et al. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci. Adv. 3, e1601314 (2017).
Park, J., Kim, M., Lee, Y., Lee, H. S. & Ko, H. Fingertip skin-inspired microstructured ferroelectric skins discriminate static/dynamic pressure and temperature stimuli. Sci. Adv. 1, e1500661 (2015).
Yamamoto, Y. et al. Printed multifunctional flexible device with an integrated motion sensor for health care monitoring. Sci. Adv. 2, e1601473 (2016).
Sekitani, T. et al. A rubberlike stretchable active matrix using elastic conductors. Science 321, 1468–1472 (2008).
Honda, W., Arie, T., Akita, S. & Takei, K. Printable and foldable electrodes based on a carbon nanotube-polymer composite. Phys. Status Solidi A 211, 2631–2634 (2014).
Kim, J. et al. Battery-free, stretchable optoelectronic systems for wireless optical characterization of the skin. Sci. Adv. 2, e1600418 (2016).
Cheng, Q. et al. Folding paper-based lithium-ion batteries for higher areal energy densities. Nano. Lett. 13, 4969–4974 (2013).
Hu, L. et al. Highly conductive paper for energy-storage devices. Proc. Natl Acad. Sci. USA 106, 21490–21494 (2009).
Bariya, M., Nyein, H. Y. Y. & Javey, A. Wearable sweat sensors. Nat. Electron. 1, 160–171 (2018).
Takei, K., Honda, W., Harada, S., Arie, T. & Akita, S. Toward flexible and wearable human-interactive health-monitoring devices. Adv. Healthcare Mater. 4, 487–500 (2015).
Sawada, K., Shimada, T., Ohshina, T., Takao, H. & Ishida, M. Highly sensitive ion sensors using charge transfer technique. Sens. Actuat. B 98, 69–72 (2004).
Hizawa, T., Sawada, K., Takao, H. & Ishida, M. Fabrication of a two-dimensional pH image sensor using a charge transfer technique. Sens. Actuat. B 117, 509–515 (2006).
Gao, W. et al. Wearable microsensor array for multiplexed heavy metal monitoring of body fluids. ACS Sens. 1, 866–874 (2016).
Nakata, S., Arie, T., Akita, S. & Takei, K. Wearable, flexible, and multifunctional healthcare device with an ISFET chemical sensor for simultaneous sweat pH and skin temperature monitoring. ACS Sens. 2, 443–448 (2017).
Bandodkar, A. J., Jeerapan, I. & Wang, J. Wearable chemical sensors: present challenges and future prospects. ACS Sens. 1, 464–482 (2016).
Imani, S. et al. A wearable chemical-electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nat. Commun. 7, 11650 (2016).
Akiyama, T., Ujihira, Y., Okabe, Y., Sugano, T. & Niki, E. Ion-sensitive field-effect transistors with inorganic gate oxide for pH sensing. IEEE Trans. Elect. Devices 29, 1936–1941 (1982).
Bousse, L. & Bergveld, P. The role of buried OH sites in the response mechanism of inorganic-gate pH-sensitive ISFETs. Sens. Actuat. 6, 65–78 (1984).
Nyein, H. Y. et al. A wearable electrochemical platform for noninvasive simultaneous monitoring of Ca2+ and pH. ACS Nano. 10, 7216–7224 (2016).
Lee, D. & Cui, T. Low-cost, transparent, and flexible single-walled carbon nanotube nanocomposite based ion-sensitive field-effect transistors for pH/glucose sensing. Biosens. Bioelectron. 25, 2259–2264 (2010).
Yan, C., Wang, J. & Lee, P. S. Stretchable graphene thermistor with tunable thermal index. ACS Nano. 9, 2130–2137 (2015).
Martin, A. et al. Epidermal microfluidic electrochemical detection system: enhanced sweat sampling and metabolite detection. ACS Sens. 2, 1860–1868 (2017).
Lee, Y. K. et al. Kinetics and chemistry of hydrolysis of ultrathin, thermally grown layers of silicon oxide as biofluid barriers in flexible electronic systems. ACS Appl. Mater. Interf. 9, 42633–42638 (2017).
Acknowledgements
This work was partially supported by a JSPS KAKENHI grant (17H04926), a JST PRESTO grant (JPMJPR17J5) and the TEPCO Memorial Foundation.
Author information
Authors and Affiliations
Contributions
S.N. and K.T. conceived the idea and designed the experiments. S.N., M.S., Y.F. and K.T. carried out the device fabrication and characterization. All authors contributed to analysing the data. K.T. wrote the paper and all authors provided feedback.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–12
Rights and permissions
About this article
Cite this article
Nakata, S., Shiomi, M., Fujita, Y. et al. A wearable pH sensor with high sensitivity based on a flexible charge-coupled device. Nat Electron 1, 596–603 (2018). https://doi.org/10.1038/s41928-018-0162-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41928-018-0162-5
This article is cited by
-
Material and structural considerations for high-performance electrodes for wearable skin devices
Communications Materials (2024)
-
Wide-range soft anisotropic thermistor with a direct wireless radio frequency interface
Nature Communications (2024)
-
Metal oxide ion gated transistors based sensors
Science China Technological Sciences (2024)
-
A swallowable X-ray dosimeter for the real-time monitoring of radiotherapy
Nature Biomedical Engineering (2023)
-
Hydrophobic ionic liquid-modified graphene via fluid-dynamic process for ion-to-electron transducers for all-solid-state potentiometric sensors
Carbon Letters (2023)