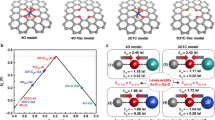
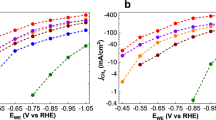
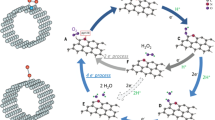
Abstract
The electrocatalytic oxygen reduction and evolution of molecular oxygen, known as oxygen electrocatalysis, is one of the most important reactions that are central to a range of energy and environmental technologies. While the current best-performing electrocatalysts remain dominated by precious metals, carbon-based systems provide a compelling alternative owing to their intrinsic sustainability and practical applicability. Here we show a design guided by theoretical calculations that pushes the activity boundaries of carbon electrocatalysts to an unprecedented level. The rationale is that incorporating high-entropy heteroatoms could effectively minimize the local symmetry to destabilize the π-electron network of graphitic carbons and avoid too strong or too weak binding energies for intermediate species of the oxygen reduction reaction and the oxygen evolution reaction. Accordingly, our catalyst embeds five metal single atoms—Fe, Mn, Co, Ni and Cu—and two sources of N, and it exhibits superior bifunctional activities in an alkaline environment that exceed the oxygen reduction reaction and evolution reaction performance of commercial Pt/C and RuO2 catalysts, respectively. Our work establishes electrocatalyst design principles that could open the door to sustainable solutions for critical green technologies such as fuel cells, batteries and water splitting.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data that support the findings in the current study are available within the Article and its Supplementary Information. Source data are available from the corresponding authors upon reasonable request.
References
Chen, G., Bare, S. R. & Mallouk, T. E. Development of supported bifunctional electrocatalysts for unitized regenerative fuel cells. J. Electrochem. Soc. 149, A1092–A1099 (2002).
Wang, Y., Leung, D. Y. C., Xuan, J. & Wang, H. A review on unitized regenerative fuel cell technologies, part-A: unitized regenerative proton exchange membrane fuel cells. Renew. Sustain. Energy Rev. 65, 961–977 (2016).
Wang, Z.-L., Xu, D., Xu, J.-J. & Zhang, X.-B. Oxygen electrocatalysts in metal–air batteries: from aqueous to nonaqueous electrolytes. Chem. Soc. Rev. 43, 7746–7786 (2014).
Wu, M. et al. Rational design of multifunctional air electrodes for rechargeable Zn–air batteries: recent progress and future perspectives. Energy Storage Mater. 21, 253–286 (2019).
Dong, F. et al. Atomically dispersed transition metal–nitrogen–carbon bifunctional oxygen electrocatalysts for zinc–air batteries: recent advances and future perspectives. Nanomicro Lett. 14, 36 (2021).
Zhang, S. et al. Advanced noncarbon materials as catalyst supports and non-noble electrocatalysts for fuel cells and metal–air batteries. Electrochem. Energy Rev. 4, 336–381 (2021).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017).
Xu, J. et al. Organic wastewater treatment by a single-atom catalyst and electrolytically produced H2O2. Nat. Sustain. 4, 233–241 (2021).
Kim, B. et al. A theoretical framework for oxygen redox chemistry for sustainable batteries. Nat. Sustain. 5, 708–716 (2022).
Man, I. C. et al. Universality in oxygen evolution electrocatalysis on oxide surfaces. ChemCatChem 3, 1159–1165 (2011).
Song, J. et al. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 49, 2196–2214 (2020).
Chen, Y. et al. Enhanced oxygen evolution over dual corner-shared cobalt tetrahedra. Nat. Commun. 13, 5510 (2022).
Sun, Y. et al. Covalency competition dominates the water oxidation structure–activity relationship on spinel oxides. Nat. Catal. 3, 554–563 (2020).
Zhao, S., Yang, Y. & Tang, Z. Insight into structural evolution, active sites, and stability of heterogeneous electrocatalysts. Angew. Chem. 134, e202110186 (2022).
Gorlin, Y. & Jaramillo, T. F. A bifunctional nonprecious metal catalyst for oxygen reduction and water oxidation. J. Am. Chem. Soc. 132, 13612–13614 (2010).
Yan, D. et al. Defect chemistry of nonprecious-metal electrocatalysts for oxygen reactions. Adv. Mater. 29, 1606459 (2017).
Luo, M. et al. PdMo bimetallene for oxygen reduction catalysis. Nature 574, 81–85 (2019).
Zhao, A. et al. Spinel Mn–Co oxide in N-doped carbon nanotubes as a bifunctional electrocatalyst synthesized by oxidative cutting. J. Am. Chem. Soc. 136, 7551–7554 (2014).
Zhong, H. et al. In situ anchoring of Co9S8 nanoparticles on N and S co-doped porous carbon tube as bifunctional oxygen electrocatalysts. NPG Asia Mater. 8, e308 (2016).
Zhou, C. et al. Superdurable bifunctional oxygen electrocatalyst for high-performance zinc–air batteries. J. Am. Chem. Soc. 144, 2694–2704 (2022).
Maiyalagan, T., Jarvis, K. A., Therese, S., Ferreira, P. J. & Manthiram, A. Spinel-type lithium cobalt oxide as a bifunctional electrocatalyst for the oxygen evolution and oxygen reduction reactions. Nat. Commun. 5, 3949 (2014).
Zhao, Y. et al. Few-layer graphdiyne doped with sp-hybridized nitrogen atoms at acetylenic sites for oxygen reduction electrocatalysis. Nat. Chem. 10, 924–931 (2018).
Sun, J. et al. Molecular engineering of Ni–/Co–porphyrin multilayers on reduced graphene oxide sheets as bifunctional catalysts for oxygen evolution and oxygen reduction reactions. Chem. Sci. 7, 5640–5646 (2016).
Zhang, J., Zhao, Z., Xia, Z. & Dai, L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nanotechnol. 10, 444–452 (2015).
Guo, D. et al. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 351, 361–365 (2016).
Jia, Y. et al. Identification of active sites for acidic oxygen reduction on carbon catalysts with and without nitrogen doping. Nat. Catal. 2, 688–695 (2019).
Chen, B., Zhong, X., Zhou, G., Zhao, N. & Cheng, H. Graphene-supported atomically dispersed metals as bifunctional catalysts for next-generation batteries based on conversion reactions. Adv. Mater. 34, 2105812 (2022).
Meng, F., Zhong, H., Yan, J. & Zhang, X. Iron-chelated hydrogel-derived bifunctional oxygen electrocatalyst for high-performance rechargeable Zn–air batteries. Nano Res. 10, 4436–4447 (2017).
Jiao, L. et al. Chemical vapour deposition of Fe–N–C oxygen reduction catalysts with full utilization of dense Fe–N4 sites. Nat. Mater. 20, 1385–1391 (2021).
Xia, C. et al. General synthesis of single-atom catalysts with high metal loading using graphene quantum dots. Nat. Chem. 13, 887–894 (2021).
Zhao, C.-X. et al. A clicking confinement strategy to fabricate transition metal single-atom sites for bifunctional oxygen electrocatalysis. Sci. Adv. 8, eabn5091 (2022).
Yu, H. et al. Nitrogen-doped porous carbon nanosheets templated from g-C3N4 as metal-free electrocatalysts for efficient oxygen reduction reaction. Adv. Mater. 28, 5080–5086 (2016).
Sun, J. et al. Ultrathin nitrogen-doped holey carbon@graphene bifunctional electrocatalyst for oxygen reduction and evolution reactions in alkaline and acidic media. Angew. Chem. Int. Ed. 57, 16511–16515 (2018).
Ma, T. Y., Dai, S., Jaroniec, M. & Qiao, S. Z. Graphitic carbon nitride nanosheet–carbon nanotube three-dimensional porous composites as high-performance oxygen evolution electrocatalysts. Angew. Chem. Int. Ed. 126, 7409–7413 (2014).
Zheng, Y. et al. Rational design of common transition metal–nitrogen–carbon catalysts for oxygen reduction reaction in fuel cells. Nano Energy 30, 443–449 (2016).
Mehmood, A. et al. High loading of single atomic iron sites in Fe–NC oxygen reduction catalysts for proton exchange membrane fuel cells. Nat. Catal. 5, 311–323 (2022).
Ji, B. et al. Metalloid-cluster ligands enabling stable and active FeN4–Ten motifs for oxygen reduction reaction. Adv. Mater. 34, 2202714 (2022).
Wu, G., More, K. L., Johnston, C. M. & Zelenay, P. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt. Science 332, 443–447 (2011).
Chen, J. et al. Dual single-atomic Ni–N4 and Fe–N4 sites constructing janus hollow graphene for selective oxygen electrocatalysis. Adv. Mater. 32, 2003134 (2020).
Bai, L., Hsu, C.-S., Alexander, D. T. L., Chen, H. M. & Hu, X. Double-atom catalysts as a molecular platform for heterogeneous oxygen evolution electrocatalysis. Nat. Energy 6, 1054–1066 (2021).
Yang, G. et al. Regulating Fe-spin state by atomically dispersed Mn–N in Fe–N–C catalysts with high oxygen reduction activity. Nat. Commun. 12, 1734 (2021).
Wang, T., Chen, H., Yang, Z., Liang, J. & Dai, S. High-entropy perovskite fluorides: a new platform for oxygen evolution catalysis. J. Am. Chem. Soc. 142, 4550–4554 (2020).
Cui, M. et al. High-entropy metal sulfide nanoparticles promise high-performance oxygen evolution reaction. Adv. Energy Mater. 11, 2002887 (2021).
Ma, Y. et al. High-entropy energy materials: challenges and new opportunities. Energy Environ. Sci. 14, 2883–2905 (2021).
Qiu, H.-J. et al. Nanoporous high-entropy alloys for highly stable and efficient catalysts. J. Mater. Chem. A 7, 6499–6506 (2019).
Liu, H. et al. High-entropy alloys and compounds for electrocatalytic energy conversion applications. SusMat 1, 482–505 (2021).
Santos, J. C., Tiznado, W., Contreras, R. & Fuentealba, P. Sigma–pi separation of the electron localization function and aromaticity. J. Chem. Phys. 120, 1670–1673 (2004).
Poater, J., Duran, M., Solà, M. & Silvi, B. Theoretical evaluation of electron delocalization in aromatic molecules by means of atoms in molecules (AIM) and electron localization function (ELF) topological approaches. Chem. Rev. 105, 3911–3947 (2005).
Lai, Q., Zhu, S., Luo, X., Zou, M. & Huang, S. Ultraviolet–visible spectroscopy of graphene oxides. AIP Adv. 2, 032146 (2012).
Ferrari, A. C. & Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 64, 075414 (2001).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B 48, 13115–13118 (1993).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Wellendorff, J. et al. Density functionals for surface science: exchange-correlation model development with Bayesian error estimation. Phys. Rev. B 85, 235149 (2012).
Blochl, P. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Acknowledgements
We acknowledge financial support from the National Key R&D Program of China (grant no. 2022YFB2402600), the National Natural Science Foundation of China (grant nos 52125105, 51902339, 52273312, 51972329 and 52173242), the Shenzhen Science and Technology Planning Project (grant nos JCYJ20200109115424940 and JCYJ20210324101015037), and the Science and Technology Planning Project of Guangdong Province (grant no. 2019TX05L389).
Author information
Authors and Affiliations
Contributions
Y.T. and Y.Z. conceived and designed the experiments and calculations. Q.T., X.L. and B.J. fabricated the samples and conducted the structure characterization and electrochemical experiments. X.L. and Y.Z. conducted the simulation work. P.K. and X.Z. performed the synchrotron-based characterizations. X.L., Y.Z. and Y.T. wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Shuhui Sun and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–29, Tables 1–5, and references 1–5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lei, X., Tang, Q., Zheng, Y. et al. High-entropy single-atom activated carbon catalysts for sustainable oxygen electrocatalysis. Nat Sustain 6, 816–826 (2023). https://doi.org/10.1038/s41893-023-01101-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-023-01101-z