Abstract
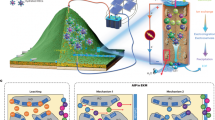
Milling minerals rich in magnesium and iron within CO2 gas has been proposed to capture carbon as metal-carbonates. We conduct milling experiments in CO2 and show that polymineralic rocks such as granite and basalt, whether high or low in carbonate-forming metals, are more efficient at trapping CO2 than individual minerals. This is because the trapping process is not, as previously thought, based on the carbonation of carbonate-forming metals. Instead, CO2 is chemically adsorbed into the crystal structure, predominantly at the boundaries between different minerals. Leaching experiments on the milled mineral/rock powders show that CO2 trapped in single minerals is mainly soluble, whereas CO2 trapped in polymineralic rocks is not. Under ambient temperature conditions, polymineralic rocks can capture >13.4 mgCO2 g−1 as thermally stable, insoluble CO2. Polymineralic rocks are crushed worldwide to produce construction aggregate. If crushing processes could be conducted within a stream of effluent CO2 gas (as produced from cement manufacture), our findings suggest that for every 100 Mt of hard rock aggregate sold, 0.4–0.5 MtCO2 could be captured as a by-product.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data that support the findings in this paper are available within the article and its Supplementary Information.
References
Lackner, K. S., Wendt, C. H., Butt, D. P., Joyce, E. L. & Sharp, D. H. Carbon dioxide disposal in carbonate minerals. Energy 20, 1153–1170 (1995).
Lackner, K. S., Butt, D. P. & Wendt, C. H. Progress on binding CO2 in mineral substrates. Energy Convers. Manage. 38, S259–S264 (1997).
Rigopoulos, I. et al. Carbon dioxide storage in olivine basalts: effect of ball milling process. Powder Technol. 273, 220–229 (2015).
Rigopoulos, I. et al. Enhancing the rate of ex situ mineral carbonation in dunites via ball milling. Adv. Powder Technol. 27, 360–371 (2016).
Rigopoulos, I., Delimitis, A., Ioannou, I., Efstathiou, A. M. & Kyratsi, E, T. Effects of ball milling on the carbon sequestration efficiency of serpentinized peridotites. Miner. Eng. 120, 66–74 (2018).
Rigopoulos, I. et al. On the potential use of quarry waste material for CO2 sequestration. J. CO2 Util. 16, 361–370 (2016).
Liu, X. et al. A review on mechanochemistry: approaching advanced energy materials with greener force. Adv. Mater. 34, 2108327 (2022).
Sanna, A. L. et al. Chemical effects induced by the mechanical processing of granite powder. Sci. Rep. 12, 9445 (2022).
Torre, F. et al. Room temperature hydrocarbon generation in olivine powders: effect of mechanical processing under CO2 atmosphere. Powder Technol. 364, 915–923 (2020).
Gamba, N., Farina, V., Garroni, S., Mulas, G. & Gennari, F. CO2 storage and conversion to CH4 by wet mechanochemical activation of olivine at room temperature. Powder Technol. 377, 857–867 (2021).
Kalinkin, A. M. Kinetics of carbon dioxide mechanosorption by Ca-containing silicates: CO2 released on heating of mechanochemically activated samples. J. Therm. Anal. Calorim. 95, 105–110 (2009).
Nelson, M. G. Carbon Dioxide Sequestration by Mechanochemical Carbonation of Mineral Silicates (UNT Digital Library, accessed 1 February 2023); https://digital.library.unt.edu/ark:/67531/metadc779600/
Kalinkin, A. M., Kalinkina, E. V. & Zalkind, O. A. Mechanosorption of carbon dioxide by Ca- and Mg-containing silicates and alumosilicates. Sorption of CO2 and structure-related chemical changes. Colloid J. 71, 185–192 (2009).
Sandvik, K. L., Kleiv, R. A. & Haug, T. A. Mechanically activated minerals as a sink for CO2. Adv. Powder Technol. 22, 416–421 (2011).
Li, J. & Hitch, M. Mechanical activation of magnesium silicates for mineral carbonation, a review. Miner. Eng. 128, 69–83 (2018).
Siriwardane, R. V., Shen, M.-S. & Fisher, E. P. Adsorption of CO2, N2 and O2 on natural zeolites. Energy Fuels 17, 571–576 (2003).
Olszak-Humienik, M. & Jablonski, M. Thermal behavior of natural dolomite. J. Therm. Anal. Calorim. 119, 2239–2248 (2015).
Erkey, C. & Türk, M. (eds) in Supercritical Fluid Science and Technology Vol . 8, Ch. 6, 73–127 (Elsevier, 2021).
Stillings, M. et al. Microseismic events cause significant pH drops in groundwater. Geophys. Res. Lett. 48, e2020GL089885 (2021).
Telling, J. et al. Rock comminution as a source of hydrogen for subglacial ecosystems. Nat. Geosci. 8, 851–855 (2015).
Kalinkin, A. M., Kalinkina, E. V., Zalkind, O. A. & Makarova, T. I. Mechanochemical interaction of alkali metal metasilicates with carbon dioxide: 1. Absorption of CO2 and phase formation. Colloid J. 70, 33–41 (2008).
Herwegh, M., Linckens, J., Ebert, A., Berger, A. & Brodhag, S. H. The role of second phases for controlling microstructural evolution in polymineralic rocks: a review. J. Struct. Geol. 33, 1728–1750 (2011).
Moir, H., Lunn, R. J., Micklethwaite, S. & Shipton, Z. K. Distant off-fault damage and gold mineralization: the impact of rock heterogeneity. Tectonophysics 608, 461–467 (2013).
Butyagin, P. Y. Mechanochemical reactions of solids with gases. React. Solids 1, 345–359 (1986).
Mineral Resources In Norway, Production Data And Annual Report (Directorate of Mining with Commissioner of Mines at Svalbard, Geological survey of Norway, 2007); https://www.ngu.no/upload/publikasjoner/rapporter/2008/mineralresources_eng_2007.pdf
Mitchell, C. J., Mitchell, P. & Pascoe, R. D. Quarry fines minimisation: can we really have 10mm aggregate with no fines? In Proc. 14th Extractive Industry Geology Conference (ed. Walton, G.) 37–44 (EIG Conferences, 2008).
Emissions to Air (Statistisk sentralbyrå (Statistics Norway), 2022); https://www.ssb.no/en/natur-og-miljo/forurensning-og-klima/statistikk/utslipp-til-luft (2022).
Kartini, K., Hamidah, M. S., Norhana, A. R. & Nur Hanani, A. R. Quarry dust fine powder as substitute for ordinary Portland cement in concrete mix. J. Eng. Sci. Technol. 9, 191–205 (2014).
Acknowledgements
This work was part-funded by the Engineering and Physical Sciences Research Council’s Doctoral Training Awards Grant EP/M506643/1. R.J.L. is funded by a Royal Academy of Engineering Research Chair. We thank W. Sloan for his helpful input on the manuscript. For the purpose of open access, the authors have applied a Creative Commons Attribution (CC BY) licence to any Author Accepted Manuscript version arising.
Author information
Authors and Affiliations
Contributions
M.S. and R.J.L. conceived the concept for the article and designed the experiments. M.S. performed the experiments. Results were analysed and interpreted by M.S., R.J.L. and Z.K.S. R.J.L. supervised the research. M.S. and R.J.L. co-wrote the manuscript with significant contributions from Z.K.S. All authors discussed the results, commented on and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Ioannis Rigopoulos, Allan Scott and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–4 and Tables 1–7.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stillings, M., Shipton, Z.K. & Lunn, R.J. Mechanochemical processing of silicate rocks to trap CO2. Nat Sustain 6, 780–788 (2023). https://doi.org/10.1038/s41893-023-01083-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-023-01083-y
This article is cited by
-
Most rocks trap CO2
Nature Sustainability (2023)