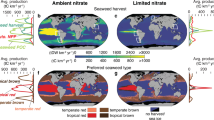
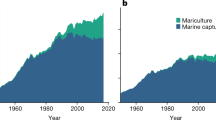
Abstract
Agricultural expansion to meet humanity’s growing needs for food and materials is a leading driver of land-use change, exacerbating climate change and biodiversity loss. Seaweed biomass farmed in the ocean could help reduce demand for terrestrial crops and reduce agricultural greenhouse gas emissions by providing a substitute or supplement for food, animal feed and biofuels. Here we model the global expansion potential of seaweed farming and explore how increased seaweed utilization under five different scenarios that consider dietary, livestock feed and fuel production seaweed usage may affect the environmental footprint of agriculture. For each scenario, we estimate the change in environmental impacts on land from increased seaweed adoption and map plausible marine farming expansion on the basis of 34 commercially important seaweed species. We show that ~650 million hectares of global ocean could support seaweed farms. Cultivating Asparagopsis for ruminant feed provided the highest greenhouse gas mitigation of the scenarios considered (~2.6 Gt CO2e yr−1). Substituting human diets at a rate of 10% globally is predicted to spare up to 110 million hectares of land. We illustrate that global production of seaweed has the potential to reduce the environmental impacts of terrestrial agriculture, but caution is needed to ensure that these challenges are not displaced from the land to the ocean.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data that support the findings of this study are from publicly available datasets, including the following:
1. Macroalgal Herbarium Portal Collections search results, September 2022 (https://macroalgae.org/portal/collections/list. php).
2. Ocean Biodiversity Information System (OBIS), September 2022 (http://www.obis.org).
3. GBIF.org occurrence download, September 2022 (https://doi.org/10.15468/dl.bd74c9).
4. GBIF.org occurrence download, September 2022 (https://doi.org/10.15468/dl.khbhnz).
5. GBIF.org occurrence download, September 2022 (https://doi.org/10.15468/dl.gwsja7).
6. Atlas of Living Australia occurrence download, 2022 (https://doi.org/10.26197/ala.9a9f5159-2c05-4949-80be-b14ecbc80061).
7. Atlas of Living Australia occurrence download, 2022 (https://doi.org/10.26197/ala.42c6ed7d-fb85-4b09-ac09-5b5c910babff).
8. Atlas of Living Australia occurrence download, 2022 (https://doi.org/10.26197/ala.5fb60371-cf43-43e0-812a-2a75f5c5c704).
Additional data are available at https://zenodo.org/deposit/7374943.
Code availability
The code that supports the findings of this study is available at https://zenodo.org/deposit/7374943.
References
Alleway, H. K. et al. The ecosystem services of marine aquaculture: valuing benefits to people and nature. BioScience 69, 59–68 (2019).
Theuerkauf, S. J. et al. Habitat value of bivalve shellfish and seaweed aquaculture for fish and invertebrates: pathways, synthesis and next steps. Rev. Aquac. https://doi.org/10.1111/raq.12584 (2021).
Duarte, C. M., Bruhn, A. & Krause-Jensen, D. A seaweed aquaculture imperative to meet global sustainability targets. Nat. Sustain. https://doi.org/10.1038/s41893-021-00773-9 (2021).
Gentry, R. R. et al. Exploring the potential for marine aquaculture to contribute to ecosystem services. Rev. Aquac. https://doi.org/10.1111/raq.12328 (2019).
Oyinlola, M. A., Reygondeau, G., Wabnitz, C. C. C., Troell, M. & Cheung, W. W. L. Global estimation of areas with suitable environmental conditions for mariculture species. PLoS ONE 13, e0191086 (2018).
Froehlich, H. E., Runge, C. A., Gentry, R. R., Gaines, S. D. & Halpern, B. S. Comparative terrestrial feed and land use of an aquaculture-dominant world. Proc. Natil Acad. Sci. USA 115, 5295–5300 (2018).
Lindsey White, W. & Wilson, P. in Seaweed Sustainability (eds Tiwari, B. K. & Declan, T. J.) 7–25 (Elsevier, 2015).
Thomas, J. B. E., Ramos, F. S. & Grondahl, F. Identifying suitable sites for macroalgae cultivation on the Swedish west coast. Coast. Manage. 47, 88–106 (2019).
Sarker, S. et al. Spatial prediction of seaweed habitat for mariculture in the coastal area of Bangladesh using a Generalized Additive Model. Algal Res. https://doi.org/10.1016/j.algal.2021.102490 (2021).
Lehahn, Y., Ingle, K. N. & Golberg, A. Global potential of offshore and shallow waters macroalgal biorefineries to provide for food, chemicals and energy: feasibility and sustainability. Algal Res. 17, 150–160 (2016).
Froehlich, H. E., Afflerbach, J. C., Frazier, M. & Halpern, B. S. Blue growth potential to mitigate climate change through seaweed offsetting. Curr. Biol. 29, 3087–3093.e3 (2019).
World Bank Group. Seaweed Aquaculture for Food Security, Income Generation and Environmental Health in Tropical Developing Countries Technical Report (World Bank, December 2016).
Antoine de Ramon, N.-Y., Chynoweth, D. P., Capron, M. E., Stewart, J. R. & Hasan, M. A. Negative carbon via ocean afforestation. Process Saf. Environ. Prot. 90, 467–474 (2012).
Sondak, C. F. A. et al. Carbon dioxide mitigation potential of seaweed aquaculture beds (SABs). J. Appl. Phycol. 29, 2363–2373 (2017).
Forster, J. & Radulovich, R. in Seaweed Sustainability: Food and Non-Food Applications (eds Tiwari, B. K. & Declan, T. J.) 289–313 (Forster Consulting, 2015).
Havlík, P. et al. Global land-use implications of first and second generation biofuel targets. Energy Policy 39, 5690–5702 (2011).
Allen, E., Wall, D. M., Herrmann, C., Xia, A. & Murphy, J. D. What is the gross energy yield of third generation gaseous biofuel sourced from seaweed? Energy 81, 352–360 (2015).
Ramachandra, T. V. & Hebbale, D. Bioethanol from macroalgae: prospects and challenges. Renew. Sustain. Energy Rev. 117, 109479 (2020).
Whiting, J. M. et al. Simulating the trajectory and biomass growth of free-floating macroalgal cultivation platforms along the U.S. west coast. J. Mar. Sci. Eng. 8, 938 (2020).
Buschmann, A. H. et al. Seaweed production: overview of the global state of exploitation, farming and emerging research activity. Eur. J. Phycol. 52, 391–406 (2017).
Syfert, M. M., Smith, M. J. & Coomes, D. A. The effects of sampling bias and model complexity on the predictive performance of MaxEnt species distribution models. PLoS ONE 8, e55158 (2013).
Assis, J. et al. Bio-ORACLE v2.0: extending marine data layers for bioclimatic modelling. Glob. Ecol. Biogeogr. 27, 277–284 (2018).
Kübler, J. E. et al. Climate change challenges and opportunities for seaweed aquaculture in California, the United States. J. World Aquac. Soc. 52, 1069–1080 (2021).
Skrzypczyk, V. M. et al. Is Australian seaweed worth eating? Nutritional and sensorial properties of wild-harvested Australian versus commercially available seaweeds. J. Appl. Phycol. 31, 709–724 (2019).
Roque, B. M. et al. Red Seaweed (Asparagopsis taxiformis) Supplementation Reduces Enteric Methane by Over 80 Percent in Beef Steers Technical Report (Systems Biology, 2020).
Fresán, U. & Sabaté, J. Vegetarian diets: planetary health and its alignment with human health. Adv. Nutr. 10, S380–S388 (2019).
Liu, Z., Deng, Z., Davis, S. J., Giron, C. & Ciais, P. Monitoring global carbon emissions in 2021. Nat. Rev. Earth Environ. 3, 217–219 (2022).
Andrew, R. & Peters, G. The Global Carbon Project’s Fossil CO2 Emissions Dataset (The Global Carbon Project, 2021); https://doi.org/10.6084/m9.figshare.16729084.v1
Glasson, C. R. K. et al. Benefits and risks of including the bromoform containing seaweed Asparagopsis in feed for the reduction of methane production from ruminants. Algal Res. 64, 102673 (2022).
Duarte, C. M., Wu, J., Xiao, X., Bruhn, A. & Krause-Jensen, D. Can seaweed farming play a role in climate change mitigation and adaptation? Front. Mar. Sci. https://doi.org/10.3389/fmars.2017.00100 (2017).
Manochio, C., Andrade, B. R., Rodriguez, R. P. & Moraes, B. S. Ethanol from biomass: a comparative overview. Renew. Sustain. Energy Rev. 80, 743–755 (2017).
Mancuso, F. P., D’Agostaro, R., Milazzo, M. & Chemello, R. The invasive Asparagopsis taxiformis hosts a low diverse and less trophic structured molluscan assemblage compared with the native Ericaria brachycarpa. Mar. Environ. Res. 166, 105279 (2021).
Onwezen, M. C., Bouwman, E. P., Reinders, M. J. & Dagevos, H. A systematic review on consumer acceptance of alternative proteins: pulses, algae, insects, plant-based meat alternatives, and cultured meat. Appetite 159, 105058 (2021).
Costa, M., Cardoso, C., Afonso, C., Bandarra, N. M. & Prates, J. A. M. Current knowledge and future perspectives of the use of seaweeds for livestock production and meat quality: a systematic review. J. Anim. Physiol. Anim. Nutr. https://doi.org/10.1111/jpn.13509 (2021).
Jiang, R., Ingle, K. N. & Golberg, A. Macroalgae (seaweed) for liquid transportation biofuel production: what is next? Algal Res. 14, 48–57 (2016).
Torres, M. D., Kraan, S. & Domínguez, H. Seaweed biorefinery. Rev. Environ. Sci. Biotechnol. 18, 335–388 (2019).
Reid, D., Mawdsley, J., Fry, J., Collins, M. & Aumonier, S. Environmental Assessment: Life Cycle Assessment Of Biofuels From Seaweed Using The Macrofuels Concept Technical Report. MacroFuels Project. H2020-LCE-11-2015 (2019).
Jouffray, J.-B., Blasiak, R., Norström, A. V., Österblom, H. & Nyström, M. The blue acceleration: the trajectory of human expansion into the ocean. One Earth 2, 43–54 (2020).
Czyrnek-Delêtre, M. M., Rocca, S., Agostini, A., Giuntoli, J. & Murphy, J. D. Life cycle assessment of seaweed biomethane, generated from seaweed sourced from integrated multi-trophic aquaculture in temperate oceanic climates. Appl. Energy 196, 34–50 (2017).
Buck, B. H., Nevejan, N., Wille, M., Chambers, M. D. & Chopin, T. in Aquaculture Perspective of Multi-Use Sites in the Open Ocean: The Untapped Potential for Marine Resources in the Anthropocene (eds. Buck, B. H. and Langan, R.) 23–69 (Marine Aquaculture, Maritime Technologies and ICZM, Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research, 2017).
Bach, L. T. et al. Testing the climate intervention potential of ocean afforestation using the Great Atlantic Sargassum Belt. Nat. Commun. https://doi.org/10.1038/s41467-021-22837-2 (2021).
Keng, F. S.-L. L. et al. The emission of volatile halocarbons by seaweeds and their response towards environmental changes. J. Appl. Phycol. 32, 1377–1394 (2020).
Krause-Jensen, D. & Duarte, C. M. Substantial role of macroalgae in marine carbon sequestration. Nat. Geosci. 9, 737–742 (2016).
Lovelock, C. E. & Duarte, C. M. Dimensions of Blue Carbon and emerging perspectives. Biol. Lett. 15, 20180781 (2019).
Troell, M., Henriksson, P. J. G., Buschmann, A. H., Chopin, T. &d Quahe, S. Farming the ocean – seaweeds as a quick fix for the climate? Rev. Fish. Sci. Aquac. https://doi.org/10.1080/23308249.2022.2048792 (2022).
Hijmans, R. J., Phillips, S., Leathwick, J. & Elith, J. (2017). dismo: Species Distribution Modeling. R package version 1.3-3. https://CRAN.R-project.org/package=dismo
Kaky, E., Nolan, V., Alatawi, A. & Gilbert, F. A comparison between Ensemble and MaxEnt species distribution modelling approaches for conservation: a case study with Egyptian medicinal plants. Ecol. Inform. 60, 101150 (2020).
Valavi, R., Guillera-Arroita, G., Lahoz-Monfort, J. J. & Elith, J. Predictive performance of presence-only species distribution models: a benchmark study with reproducible code. Ecol. Monogr. https://doi.org/10.1002/ecm.1486 (2021).
Evans, J. M., Fletcher, R. J. & Alavalapati, J. Using species distribution models to identify suitable areas for biofuel feedstock production. GCB Bioenergy 2, 63–78 (2010).
Jayathilake, D. R. M. & Costello, M. J. A modelled global distribution of the kelp biome. Biol. Conserv. 252, 108815 (2020).
Wiltshire, K. H. & Tanner, J. E. Comparing maximum entropy modelling methods to inform aquaculture site selection for novel seaweed species. Ecol. Modell. https://doi.org/10.1016/j.ecolmodel.2020.109071 (2020).
Hijmans, R. J. (2022) terra: Spatial Data Analysis. R package version 1.4-11; https://CRAN.R-project.org/package=terra
Rebecca, R. et al. Mapping the global potential for marine aquaculture. Nat. Ecol. Evol. 1, 1317–1324 (2017).
Maritime Boundaries Geodatabase: Maritime Boundaries and Exclusive Economic Zones (200NM) Version 10 (VLIZ Flanders Marine Institute, 2020); https://doi.org/10.14284/312
Campbell, I. et al. Biosecurity policy and legislation for the global seaweed aquaculture industry. J. Appl. Phycol. 32, 2133–2146 (2020).
Garcia-Oliveira, P. et al. Macroalgae as an alternative source of nutrients and compounds with bioactive potential. Proceedings 70, 46 (2020).
Tibbetts, S. M., Milley, J. E. & Lall, S. P. Nutritional quality of some wild and cultivated seaweeds: nutrient composition, total phenolic content and in vitro digestibility. J. Appl. Phycol. 28, 3575–3585 (2016).
Øverland, M., Mydland, L. T. & Skrede, A. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J. Sci. Food Agric. 99, 13–24 (2019).
Brown, E. M. et al. Seaweed and human health. Nutr. Rev. 72, 205–216 (2014).
Birch, D., Skallerud, K. & Paul, N. Who eats seaweed? An Australian perspective. J. Int. Food Agribus. Mark. 31, 329–351 (2019).
Monteiro, M. S., Sloth, J., Holdt, S. & Hansen, M. Analysis and Risk Assessment of Seaweed. EFSA J. https://doi.org/10.2903/j.efsa.2019.e170915 (2019).
Hansen, H. R., Hector, B. L. & Feldmann, J. A qualitative and quantitative evaluation of the seaweed diet of North Ronaldsay sheep. Anim. Feed Sci. Technol. 105, 21–28 (2003).
Kinley, R. D., de Nys, R., Vucko, M. J., Machado, L. & Tomkins, N. W. The red macroalgae Asparagopsis taxiformis is a potent natural antimethanogenic that reduces methane production during in vitro fermentation with rumen fluid. Anim. Prod. Sci. 56, 282–289 (2016).
Roque, B. M., Salwen, J. K., Kinley, R. & Kebreab, E. Inclusion of Asparagopsis armata in lactating dairy cows’ diet reduces enteric methane emission by over 50 percent. J. Clean. Prod. 234, 132–138 (2019).
Li, X. et al. Asparagopsis taxiformis decreases enteric methane production from sheep. Anim. Prod. Sci. 58, 681–688 (2018).
Lee, X. J., Ong, H. C., Gan, Y. Y., Chen, W.-H. & Mahlia, T. M. I. State of art review on conventional and advanced pyrolysis of macroalgae and microalgae for biochar, bio-oil and bio-syngas production. Energy Convers. Manage. 210, 112707 (2020).
Ullah, K. et al. Algal biomass as a global source of transport fuels: overview and development perspectives. Prog. Nat. Sci. Mater. Int. 24, 329–339 (2014).
Lauri, P. et al. Global woody biomass harvest volumes and forest area use under different SSP-RCP scenarios. J. For. Econ. 34, 285–309 (2019).
Newbold, T. et al. Has land use pushed terrestrial biodiversity beyond the planetary boundary? A global assessment. Science 353, 288–291 (2016).
Fricko, O. et al. The marker quantification of the Shared Socioeconomic Pathway 2: a middle-of-the-road scenario for the 21st century. Glob. Environ. Change 42, 251–267 (2017).
Acknowledgements
We thank P. Lauri (International Institute for Applied Systems Analysis) for help with modelling afforestation. Part of the research was developed in the Young Scientists Summer Program at the International Institute for Applied Systems Analysis, Laxenburg (Austria) with financial support from the United States National Member Organization. S.S. was funded by a Research Training Program Scholarship from the Australian Government.
Author information
Authors and Affiliations
Contributions
S.S. and H.V. initiated and designed the project; S.S. compiled all of the data sources and performed the species distribution modelling and GLOBIOM modelling and developed code for the spatial prioritization with key suggestions from H.V., M.B., F.S. and E.M.-M.; H.V. and M.B. provided data on the properties of commodities; H.V., P.H., F.S., R.S.C., K.R.O. and E.M.-M. provided supervision and key suggestions for the manuscript; D.L. provided code for the biodiversity metric; S.S. wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Alice Jones, Jordan Hollarsmith and Heidi Alleway for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Overall constraints.
In each cell, the constraint is a product of six constrtaints that have been normalized to be between 0 and 1. Cells with a constraint value of 1 indicate ideal conditions and no constraints. Values close to 0 indicate prohibitive conditions for seaweed farming in one or several constraint layers.
Extended Data Fig. 2 Global potential for seaweed farming.
A. The global potential for seaweed farming, shown here in dark green, represents an overlay of all suitable cells for seaweed farming, based on species distribution models from 34 commercially important species and constrained by depth, distance to the nearest port, shipping traffic, mean wave energy, the presence of marine protected areas and limiting potential to areas where each species is likely to natively occur. B. The amount of suitable space per GLOBIOM region. NB: regions that contribute less than 1% of the total global potential are omitted.
Extended Data Fig. 3 Food scenario extent and statistics.
A. Extent of ocean area required to meet the ‘Food’ scenario, B. Area in most utilized regions, C. Depth and distance to nearest port of utilized cells.
Extended Data Fig. 4 Feed scenario extent and statistics.
A. Extent of ocean area required to meet the ‘Feed’ scenario, B. Area in most utilized regions, C. Depth and distance to nearest port of utilized cells.
Extended Data Fig. 5 Fuel scenario extent and statistics.
A. Extent of ocean area required to meet the ‘Fuel’ scenario, B. Area in most utilized regions, C. Depth and distance to nearest port of utilized cells.
Extended Data Fig. 6 Aspa scenario extent and statistics.
A. Extent of ocean area required to meet the ‘Aspa’ scenario, B. Area in most utilized regions, C. Depth and distance to nearest port of utilized cells.
Extended Data Fig. 7 Regional potential to meet demand.
The potential for the world and each GLOBIOM region (See Table S1–5 for definitions) to produce enough seaweed in the waters of their EEZ to supply each of the scenarios analysed here: 10% of human diets (‘Food’), 10% of livestock diets (‘Feed’), 50% of biofuel energy (‘Fuel’), all three of the above (‘All’) and Asparagopsis for 0.5% of ruminant livestock diets (‘Aspa’).
Extended Data Fig. 8 Changes in consumption of selected products in GLOBIOM by scenario.
The changes in global consumption over the course of the GLOBIOM run (2000–2050).
Extended Data Fig. 9 Regional impacts on terrestrial biodiversity.
The regional impacts on terrestrial biodiversity (BII), compared to the baseline scenario in 2050.
Extended Data Fig. 10 Clustered nutritional profiles of terrestrial products and selected seaweeds.
A principle components analysis was used to cluster terrestrial commodities and seaweed species by energy (kcal/kg), protein (g/kg) and lipid density (g/kg). The diversity of seaweeds means that they are well distributed amongst the major terrestrial commodities and could therefore substitute for these terrestrial products on a nutritional basis. For terrestrial product definitions see Table S1–9.
Supplementary information
Supplementary Information
Supplementary Tables 1.1–1.10 and Figs. 1.1 and 1.2. Supplement 2. Supplementary Methods, Figs. 2.1–2.8 and References.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Spillias, S., Valin, H., Batka, M. et al. Reducing global land-use pressures with seaweed farming. Nat Sustain 6, 380–390 (2023). https://doi.org/10.1038/s41893-022-01043-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-022-01043-y
This article is cited by
-
Assessment of regulatory compounds in commercial red seaweed Kappaphycus alvarezii after long-distance transportation
Aquaculture International (2024)
-
Large global variations in the carbon dioxide removal potential of seaweed farming due to biophysical constraints
Communications Earth & Environment (2023)
-
Climate benefits of seaweed farming
Nature Sustainability (2023)
-
Biomass of Cladophora (Chlorophyta, Cladophorales) is a promising resource for agriculture with high benefits for economics and the environment
Aquaculture International (2023)