Abstract
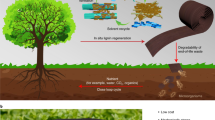
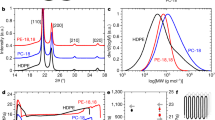
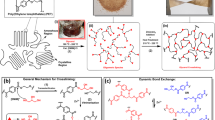
The crisis of plastic waste in the environment calls for new polymers that are designed specifically for recycling at the end of their life. Lignin, which is present in approximately 15–40% of woody biomass, is an attractive biobased source for polymers given its aromatic nature. However, the use of lignin in polymers is complicated by its own reactivity and heterogeneous structure, factors that also create difficulties for designing end-of-life solutions for lignin-based polymers. Here we demonstrate a chemical recycling technique that prevents the loss of functionality to lignin and produces a recycled precursor capable of entering back into the synthetic sequence for non-isocyanate polyurethane foams. This technique enables the depolymerization of the polymer and isolation of lignin with enhanced solubility and hydroxyl content so that it can be reused in second-generation polymers. Detailed structural analysis of lignin after chemical recycling reveals the insertion of ethylene glycol in the side-chain region during a high-pressure hydrolysis recycling procedure. The closed-loop recycling process for the lignin-derived non-isocyanate polyurethane foam demonstrates a pathway towards a circular economy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Additional data that support the findings of this study are available from the corresponding author upon request.
References
Jambeck, J. Plastic waste inputs from land into the ocean. Science 347, 768–771 (2015).
Rillig, M. C. Microplastic in terrestrial ecosystems and the soil? Environ. Sci. Technol. 46, 6453–6454 (2012).
Vanholme, R. et al. Metabolic engineering of novel lignin in biomass crops. N. Phytol. 196, 978–1000 (2012).
Upton, B. M. & Kasko, A. M. Strategies for the conversion of lignin to high-value polymeric materials: review and perspective. Chem. Rev. 116, 2275–2306 (2016).
Duval, A. & Lawoko, M. A review on lignin-based polymeric, micro- and nano-structured materials. React. Funct. Polym. 85, 78–96 (2014).
Sternberg, J., Sequerth, O. & Pilla, S. Green chemistry design in polymers derived from lignin: review and perspective. Prog. Polym. Sci. 113, 101344 (2021).
Karunarathna, M. S., Lauer, M. K., Thiounn, T., Smith, R. C. & Tennyson, A. G. Valorisation of waste to yield recyclable composites of elemental sulfur and lignin. J. Mater. Chem. A 7, 15683–15690 (2019).
Buono, P., Duval, A., Averous, L. & Habibi, Y. Thermally healable and remendable lignin-based materials through Diels–Alder click polymerization. Polym. J. 133, 78–88 (2017).
Xu, Y., Odelius, K. & Hakkarainen, M. Recyclable and flexible polyester thermosets derived from microwave-processed lignin. ACS Appl. Polym. Mater. 2, 1917–1924 (2020).
Deuss, P. J. et al. Aromatic monomers by in situ conversion of reactive intermediates in the acid-catalyzed depolymerization of lignin. J. Am. Chem. Soc. 137, 7456–7467 (2015).
Shuai, L. et al. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. Science 354, 329–333 (2016).
Lancefield, C. S., Panovic, I., Deuss, P. J., Barta, K. & Westwood, N. J. Pre-treatment of lignocellulosic feedstocks using biorenewable alcohols: towards complete biomass valorisation. Green. Chem. 19, 202–214 (2017).
Deuss, P. J. et al. Phenolic acetals from lignins of varying compositions: Via iron(iii) triflate catalysed depolymerisation. Green Chem. 19, 2774–2782 (2017).
Lancefield, C. S., Wienk, H. L. J., Boelens, R., Weckhuysen, B. M. & Bruijnincx, P. C. A. Chemical science identification of a diagnostic structural motif reveals a new reaction intermediate and condensation pathway in Kraft lignin formation. Chem. Sci. 9, 6317–6454 (2018).
Bauer, S., Sorek, H., Mitchell, V. D., Ibáñez, A. B. & Wemmer, D. E. Characterization of Miscanthus giganteus lignin isolated by ethanol Organosolv process under reflux condition. J. Agric. Food Chem. 60, 8203–8212 (2012).
Sternberg, J. & Pilla, S. Materials for the biorefinery: high bio-content, shape memory Kraft lignin-derived non-isocyanate polyurethane foams using a non-toxic protocol. Green Chem. 20, 6922–6935 (2020).
Collins, M. N. et al. Valorization of lignin in polymer and composite systems for advanced engineering applications—a review. Int. J. Biol. Macromol. 131, 828–849 (2019).
Suhas Carrot, P. J. M. & Ribeiro Carrott, M. M. L. Lignin—from natural adsorbent to activated carbon: a review. Bioresour. Technol. 98, 2301–2312 (2007).
Culebras, M. et al. Bio-derived carbon nanofibres from lignin as high-performance Li-ion anode materials. ChemSusChem 12, 4516–4521 (2019).
Motokucho, S., Nakayama, Y., Morikawa, H. & Nakatani, H. Environment-friendly chemical recycling of aliphatic polyurethanes by hydrolysis in a CO2 –water system. J. Appl. Polym. Sci. 135, 45897 (2018).
Motokucho, S., Yamaguchi, A., Nakayama, Y., Morikawa, H. & Nakatani, H. Hydrolysis of aromatic polyurethane in water under high pressure of CO2. J. Polym. Sci. A 55, 2004–2010 (2017).
Simón, D., Borreguero, A. M., de Lucas, A. & Rodríguez, J. F. Glycolysis of viscoelastic flexible polyurethane foam wastes. Polym. Degrad. Stab. 116, 23–35 (2015).
Trajano, H. L. et al. The fate of lignin during hydrothermal pretreatment. Biotechnol. Biofuels 6, 110 (2013).
Chakar, F. S. & Ragauskas, A. J. Review of current and future softwood Kraft lignin process chemistry. Ind. Crops Prod. 20, 131–141 (2004).
Granata, A. & Argyropoulos, D. S. 2-Chloro-4,4,5,5-tetramethyl-1,3>2-dioxaphospholane, a reagent for the accurate determination of the uncondensed and condensed phenolic moieties in lignins. J. Agric. Food Chem. 43, 1538–1544 (1995).
Hu, Z., Du, X., Liu, J., Chang, H. & Jameel, H. Structural characterization of pine Kraft lignin: BioChoice lignin vs Indulin AT. J. Wood Chem. Technol. 36, 432–446 (2016).
Gierer, J. Chemical aspects of Kraft pulping. Wood Sci. Technol. 14, 241–266 (1980).
Gierer, J. Chemistry of delignification. Wood Sci. Technol. 20, 1–33 (1986).
Cornille, A., Dworakowska, S., Bogdal, D., Boutevin, B. & Caillol, S. A new way of creating cellular polyurethane materials: NIPU foams. Eur. Polym. J. 66, 129–138 (2015).
Tran, F., Lancefield, C. S., Kamer, P. C. J., Lebl, T. & Westwood, N. J. Selective modification of the β–β linkage in DDQ-treated Kraft lignin analysed by 2D NMR spectroscopy. Green Chem. 17, 244–249 (2014).
Acknowledgements
We thank O. Sequerth, L. Slann and A. Devol of Clemson University for their support during this project. Research was supported by the Department of Energy, Basic Energy Sciences, award no. DE-SC0021367 (chemical recycling and characterization), by Sonoco FRESH initiative at Clemson University (NIPU synthesis) and as part of the AIM for Composites, an Energy Frontier Research Center funded by the US Department of Energy (DOE), Office of Science, Basic Energy Sciences (BES), under award no. DE-SC0023389 (NMR studies). In addition, S.P. acknowledges the Robert Patrick Jenkins Professorship. J.S. acknowledges graduate fellowship support through Cooper-Standard, Draexlmaier and Sonoco foundations.
Author information
Authors and Affiliations
Contributions
J.S. and S.P. contributed jointly to this paper. S.P. conceptualized the scope of the study and guided the research progress and manuscript editing. J.S. completed the experimental design, characterization of materials and manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Maurice Collins and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7, Tables 1–5 and Notes 1–3.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sternberg, J., Pilla, S. Chemical recycling of a lignin-based non-isocyanate polyurethane foam. Nat Sustain 6, 316–324 (2023). https://doi.org/10.1038/s41893-022-01022-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-022-01022-3
This article is cited by
-
Application and carbon footprint evaluation of lignin-based composite materials
Advanced Composites and Hybrid Materials (2024)