Abstract
Pheromones have become an environmentally friendly alternative to conventional insecticides for pest control. Most current pheromone-based pest control products target lepidopteran pests of high-value crops, as today’s manufacturing processes cannot yet produce pheromones at low enough costs to enable their use for lower-value crops, especially commodity crops. Camelina sativa seeds genetically modified to express (Z)-11-hexadecenoic acid, a sex pheromone precursor of several moth species, provided the oil from which the precursor was isolated, purified and transformed into the final pheromone. Trap lures containing this pheromone were then assessed for their capacity to manage moth pests in the field. Plant-derived pheromone lures proved equally effective as synthetic pheromone lures in monitoring the diamondback moth, Plutella xylostella, in cabbage and disrupting mating of cotton bollworm, Helicoverpa armigera, in common bean fields. Our study demonstrates the biological efficacy and economic feasibility of pheromone production in plant factories by metabolic engineering of an oilseed crop.
Similar content being viewed by others
Main
Herbivorous insects cause losses of more than one-fifth of the world’s total crop production annually1, losses projected to increase due to global warming2. Conventional chemical insecticides are extensively used for crop protection, with 400,000 tonnes of active ingredients (AIs) applied per year globally3,4, causing severe adverse impacts on ecosystems and public health5. Rapid evolution of resistance to insecticides makes pest control ever more challenging, as it requires the use of increasing doses of pesticides per treated area to achieve pest suppression5,6. For example, the Arthropod Pesticide Resistance Database (http://www.pesticideresistance.org) reports 879 cases of resistance to 52 different insecticide AIs by cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae), a worldwide key pest of row crops, and 980 cases of resistance to 101 AIs by diamondback moth, Plutella xylostella (L) (Lepidoptera: Plutellidae), a global key pest of brassicas. Public concerns about insecticide residues in food and adverse impacts of pesticides on fragile ecosystems have increased market demand for safer, more environmentally friendly bio-based pesticides1.
To address these concerns, many countries have imposed regulations aimed at reducing reliance on neuroactive insecticides for pest control7, creating an opportunity for alternative pest control technologies to gain a commercial foothold. One particularly promising strategy is the use of sex pheromones to prevent or mitigate damage by insect pests, through monitoring, mass trapping or mating disruption8,9,10. Sex pheromones are relatively species-specific chemical signals that elicit behavioural or physiological reactions in individuals of the same species. Their species specificity, often achieved by a combination of structurally similar molecules in a precise ratio, is related to their roles in mate communication and reproductive isolation11. To date, mating disruption using sex pheromones has found its readiest market in high-value horticultural fruit and nut crops but has also been put to use in forestry and stored products10.
The global market for insect pheromones in agriculture is projected to reach US$5.7 billion by 2025 and US$7.2 billion by 2027 (https://www.marketresearch.com; https://www.reportlinker.com). Adoption of mating disruption solutions for pest control in high-value specialty crops, such as fruits, vegetables and nuts, has steadily increased over the past few decades7,12,13. However, pheromone-based pest control is still rare in many economically important crops, especially row crops that are cultivated in large expanses, such as soybean, maize and cotton. This is largely due to the high cost of conventional chemical synthesis of the pheromone AIs, which puts mating disruption solutions beyond the financial reach of growers of lower-value crops12,14,15.
To lower the cost of pheromone synthesis and promote their wider use in agricultural pest management, several alternative bio-based methods of pheromone production have been evaluated, starting with proof-of-concept metabolic engineering studies conducted in bakerʼs yeast, Saccharomyces cerevisiae14,16, to modify it to produce certain moth pheromones. Use of transgenic plants to, in essence, ‘growʼ pheromone precursors or components represents an additional green chemistry alternative to synthetic pheromone production. This not only reduces the cost of these production processes but also eliminates the need for the petroleum-based and other chemical feedstocks used in conventional pheromone synthesis. In tobacco, Nicotiana benthamiana, Ding et al. transiently introduced up to four genes coding for consecutive biosynthetic steps, successfully producing multi-component sex pheromones used by two small ermine moths, Yponomeuta evonymella and Y. padella17. Mixtures of acetylated fatty alcohols derived from the tobacco leaves trapped male moths of the target species as effectively as synthetically produced pheromones17. This study clearly demonstrated the possibility of transiently producing a variety of moth pheromone components in genetically modified plants. Stable transformation of tobacco species (Nicotiana spp.) to obtain the pheromone precursor (Z)-11-hexadecenoic acid over generations in tobacco leaves was subsequently shown by Xia et al.18.
Most lepidopteran sex pheromones are mono- or di-unsaturated long straight chain (C10–C18) fatty acid derivatives with primary alcohols, acetates and aldehydes as functional groups. Many oilseed crops have prodigious biosynthetic machinery to produce high levels of fatty acids, which are stored in the seed tissues as triacylglycerols. Over the past ten years, the authors have explored the feasibility of producing moth pheromone compounds in plants at commercially relevant levels by tailoring the fatty acid composition of the oilseed crop, Camelina sativa (hereafter, Camelina), a short life cycle Brassicaceae crop that has spring and winter forms and can be cultivated in diverse climates and soil types19. Camelina produces fatty acids in concentrations of ≤40% of its seed weight and can be easily genetically transformed20, making it an attractive metabolic engineering platform for specialty seed oils for applications such as omega-3 fish oil functionality21 and renewable jet fuels22.
Here we report the metabolic engineering of Camelina for seed oil production of (Z)-11-hexadecenoic acid, the immediate fatty acid precursor of Z11-16:OH and its aldehyde and acetate derivatives. Co-expression of genes for a plant fatty acyl-acyl carrier protein thioesterase and an insect desaturase yields high production of a pheromone precursor acid in seed triacylglycerols with desired carbon-chain length and specific double bond position and configuration. We also show the feasibility of downstream seed oil processing, including a combination of simple techniques for extraction, purification and transformation of target compounds at low cost, paving the way for viable industrial-scale production of insect pheromones from plant-produced precursors. Finally, field bioassays targeting the worldwide pests, P. xylostella and H. armigera, in cabbage and common bean crops proved that Camelina seed oil-derived pheromones are as effective as conventionally synthesized pheromones in monitoring and mating disruption.
Results
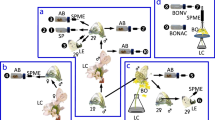
Our overall workflow, shown in Fig. 1, involved generation of Camelina transgenic lines by Agrobacterium tumefaciens-mediated floral dip transformation, identification and advance of the top-producing line, field propagation of the selected line, seed oil processing and chemical conversion of the precursor to pheromone and demonstration of the bioactivity of the Camelina-derived pheromone formulations in field monitoring and trap shutdown experiments (Fig. 1). Trap shutdown experiments measure the capacity of formulations containing sex pheromone to disrupt the ability of males to locate pheromone-lured traps in the same area. The assumption is that if males cannot locate traps containing optimized pheromone lures, which are more attractive than a calling female, then males cannot locate calling females in that same field either, indicating successful mating disruption.
a, Agrobacterium tumefaciens-mediated gene transfer by floral dip technique. b, Breeding of positively transformed lines over three generations (T1–T3) based on seed fatty acid GC/MS analysis. c, Propagation of selected lines in field trials. d, Seed oil extraction, target compound isolation and purification and chemical conversion of the precursor acid into pheromone compounds. e, Bioactivity evaluation in field trapping and trap shutdown experiments.
Generation of pheromone precursor-producing Camelina lines
A previously reported Camelina line, engineered to produce ~43% palmitic acid (16:0) by seed-specific expression of a Cuphea FatB cDNA of a 16:0-acyl-acyl carrier protein thioesterase22,23, was used as the host for seed-specific expression of the pheromone gland-specific fatty acyl-CoA desaturase Atr∆11 cDNA from the navel orangeworm, Amyelois transitella. For these experiments, the Atr∆11 cDNA was placed under control of the soybean seed-specific glycinin-1 promoter and assembled into a binary vector, with selection conferred by constitutive expression of a resistance gene for the herbicide phosphinothricin (or Basta) (Supplementary Fig. 1). Following Agrobacterium tumefaciens-mediated transformation of the high palmitic acid Camelina line with the T-DNA from the Atr∆11 cDNA-containing binary vector, phosphinothricin-resistant independent lines (namely T1) were identified, which produced T2 seeds. The T2 seeds were screened for (Z)-11-hexadecenoic acid production (Figs. 1 and 2a,b and Table 1). The average production of the target acid, Z11-16:Acid in T2 seeds from 16 positively transformed plants was 10.5 ± 0.5% (mean ± standard error of the mean (SE), weight percent throughout the text) (Fig. 3a). Large individual variation of the target acid production among T2 seeds was observed (Supplementary Fig. 2). For example, from one of the promising transformants, CpuFatB+AtrD11_#37, which produced a relatively high content of Z11-16:Acid, the percentage of Z11-16:Acid ranged from 3.5% to 24.4% among 25 individually analysed seeds. Seeds from this plant together with two other transformants, namely CpuFatB+AtrD11_#33 and CpuFatB+AtrD11_#40, were grown and selfed to obtain T3 seeds (Fig. 3b). Through this process, we obtained homozygous T4 seeds with an average of 22.1 ± 0.7% of the target acid and greatly reduced individual variation (Fig. 3c and Table 1).
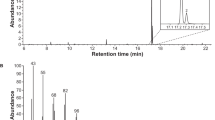
a, Representative chromatogram of fatty acid composition in seeds of a high palmitic acid-producing plant expressing a CpuFatB thioesterase, analysed by gas chromatography with a flame ionisation detector (GC-FID). b, Representative GC-FID chromatogram of fatty acid composition in T2 seeds of a CpuFatB+AtrD11 line producing the target acid Z11-16:Acid (16:1). Peaks in the chromatograms are labelled with standard abbreviations for fatty acids in the form of their methyl esters, indicating the number of the carbon atoms in the acyl chain and the level of unsaturation. For example, 14:0 stands for methyl tetradecanoate, 16:1 is methyl (Z)-11 hexadecenoate and 18:2 is methyl linoleate.
a, C16 fatty acid composition in T2 seeds from 16 positively transformed CpuFatB + AtrD11 lines; data represent pooled samples with five seeds in each and three biological replicates. b, C16 fatty acid composition in the seeds of three selected T2 lines, CpuFatB+AtrD11_#33, #37 and #40. For each line, ten plants were examined, and from each plant, 50 seeds were pooled and extracted as one sample. c, C16 fatty acid composition in the seeds of the selected T3 line CpuFatB+AtrD11_#37.2, from which ten plants were examined and 25 seeds from each plant were pooled and extracted as one sample.
Seed propagation in open fields
Depending on the region and mode of cultivation, two to three generations of Camelina may be sown and harvested in a single year. An initial propagation trial of C. sativa in open fields was performed in eastern Nebraska (USA) in 2016, sowing 15.5 g of T3 seeds from a positive transformant line. Approximately 0.3 kg of T4 seeds were produced from this field trial, containing ~9% of the target Z11-16:Acid of the total fatty acids.
Separately, T4 plants originated from CpuFatB+AtrD11_#37 were propagated in a greenhouse in Lund (Sweden) to produce T5 seeds (containing 12.7 ± 0.6% of the target acid, Z11-16:Acid) and T6 seeds (15.8 ± 1.2% Z11-16:Acid) for larger open field propagation. In 2018, 62.5 g of T5 seeds were sown in Nebraska and 121 g of T6 seeds in Borgeby, Skåne (Sweden), respectively. Propagation in Nebraska produced ~1.8 kg of T6 seeds (12.7 ± 1.7% Z11-16:Acid), whereas propagation in Borgeby produced 5.5 kg of T7 seeds (19.2 ± 0.4% Z11-16:Acid).
Seed oil processing and transformation of acid to pheromones
The field-harvested seeds containing the target acid were segregated into batches corresponding to each open field propagation trial, and the oil of each batch was extracted and purified. The fatty acid components of the crude seed oil were first converted into their corresponding methyl esters and separated based on carbon-chain length via short-path distillation (Fig. 4a). This procedure removed the majority of C18 and longer compounds, especially those with full saturation and monounsaturation. The short-path distillates were then subjected to urea complexation24 to remove most of the remaining saturated components (Fig. 4b).
a, Fatty acid composition in seed oil of transformed Camelina before and after short-path distillation. The inset photo shows the starting FAME material for short-path distillation on a scale of 1 kg in a 2.5 L flask. b, Compositions of saturated and unsaturated C16 acids in the starting FAME sample, complex and filtrates after first and second urea complexations.
Batch 1. From the 0.3 kg of T4 seed material from the Nebraska 2016 open field propagation, ~60 g fatty acid methyl esters (FAMEs) were obtained, with 9.0% Z11-16:Acid. After short-path distillation and urea complexation, AgNO3-silica gel column chromatography was applied to produce a fraction containing 38% Z11-16:Acid. An aliquot of this fraction was further fractionated on argentation thin layer chromatography (TLC) to produce a second fraction containing 83% Z11-16:Acid. The samples were subsequently reduced to corresponding alcohols and then converted to aldehydes and acetates (Supplementary Fig. 3) for trapping/monitoring activity field assay.
Batch 2. Another batch of seed oil was obtained from a combination of 1.8 kg T6 seeds from Nebraska and 2.5 kg T7 seeds from Skåne. Approximately 1 kg of FAMEs was obtained after base methanolysis, and subsequently, 240 g of the short-path distillates containing most of the C16 components were subjected to urea complexation. After urea complexation, a total of 182 g FAMEs containing ~82 g of Z11-16:Acid was obtained in two purity levels, 40% and 65%, with some non-target common fatty acids as the major impurities. From this batch, ~144 g FAMEs were used for reduction, resulting in 126 g of corresponding alcohols, and out of that, 73 g alcohols were converted into aldehydes, which resulted in a total of 30 g of the AI Z11-16:Ald at two purity levels, 45% and 75%, for the trap shutdown activity field assay (Supplementary Fig. 4).
Field activity evaluation
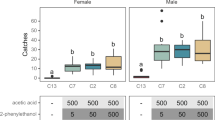
Pheromone from Batch 1 was assessed for its field monitoring trap activity against P. xylostella, and pheromone from Batch 2 was tested in a field trap shutdown trial with H. armigera. The activity of plant-derived pheromone for trapping of P. xylostella, a global pest of the genus Brassica, was tested in early 2017 in a choy sum field (Brassica rapa var. parachinensis) in Guangzhou, China. The sex pheromone of this species is a mixture of (Z)-11-hexadecenal (Z11-16:Al), (Z)-11-hexadecenyl acetate (Z11-16: OAc) and (Z)-11-hexadecenyl alcohol (Z11-16:OH), with a variable blend ratio among different geographical populations of the insect25,26,27. The plant-derived pheromones from Batch 1 were maintained at two purity levels, high (83%) and low (38%). Each purity level was adjusted to replicate the sex pheromone component blend ratio for the southern China population, a 70:30:0.1 mixture of acetate, aldehyde and alcohol27, and formulated into rubber septa (100 µg pheromone per septum). In the same experiment, we also used conventionally synthesized pheromone at the same ratio and dose formulated into rubber septa as a positive control, and the solvent n-heptane alone formulated into rubber septa as a negative control. This study assessed the capacity of lures containing plant-derived pheromone at two levels of purity to capture male P. xylostella compared with lures using high-purity synthetic pheromone (Supplementary Fig. 3 and Fig. 5a). No significant differences were observed in average number of male moths captured per trap per week for weeks 1, 2, 4, 6 and 7 for all pheromone-lured traps, independent of the origin of the pheromone. However, catches by synthetic pheromone-lure traps were numerically slightly larger. Except for week 8, all pheromone-baited traps had significantly higher male trap catches than those baited with a solvent control. Interestingly, traps baited with the lower purity of plant-derived pheromone (38%) performed as well as those baited with synthetic pheromone lures throughout the first seven weeks of the monitoring experiment. The data from this B. rapa field monitoring study indicated that P. xylostella males do not discriminate between plant-derived and synthetic-derived pheromone lures. It also suggests that the impurities found in plant-derived pheromone lures are not detrimental to the attraction and capture of P. xylostella males in monitoring traps.
a, Weekly catches (mean number ± standard error of the mean (SE)) of male diamondback moth, Plutella xylostella, during eight weeks in three fields of Brassica rapa in southern China (W1–W8, 8 March to 3 May, 2017). N = 5 for each treatment group. Individual data values (black dots) were shown along with the chart. Bars with the same letters indicate catches that are not significantly different among treatments within the same week (one-way ANOVA followed by LSD test at 0.05 level). During the experimental period, the lures were replaced once by the end of week 6. b, Trap catches in plots treated for mating disruption of the cotton bollworm Helicoverpa armigera in a field of beans in Brazil. Bars indicate the trap catch after treatment with synthetic or plant-derived pheromone of different purities, each containing 1.8 g of the AI (Z11-16:Ald) per plot (Supplementary Fig. 4) (26 September to 28 October, 2020). N = 4 for each treatment group. Individual data values (black dots) are shown along with the chart. Bars with the same letters indicate treatments that are not significantly different (one-way ANOVA followed by Tukeyʼs test at 0.05 level).
To verify the mating disruption field activity of formulations containing the plant-derived pheromone, Z11-16:Ald, a trap shutdown experiment was performed in a field of common bean, Phaseolus vulgaris, in São Paulo, Brazil, targeting the noctuid species, H. armigera, a global agricultural pest of row crops that uses Z11-16:Ald as its major sex pheromone component28. Two purity levels of the plant-derived pheromone were assessed, 45% and 75% (from Batch 2), compared with that of high-purity synthetic pheromone (≥98%). The results showed that when compared to those of the negative control treatment, the presence of the tested mating disruption formulation had a clear suppressive effect on male H. armigera captures in every one of the three pheromone treatments. Efficacy did not vary significantly between mating disruption treatments using pheromone derived from either source (synthetically produced or plant-derived), nor between those with higher or lower purity of plant-derived pheromones (45% or 75%) (Fig. 5b). The latter finding suggests that impurities found in plant-derived pheromone do not decrease efficacy of mating disruption of H. armigera.
Discussion
With growing concerns over the sustainability of chemical pesticide use, safe and eco-friendly alternatives are needed to preserve crop yield, quality and food security. A promising alternative to toxic pesticides is the use of pheromones to suppress pest populations in the field. Despite decades of research demonstrating the efficacy of pheromone-based pest control, their adoption has been limited to high-value niche markets because of the high cost of the pheromone AIs29 but also due to availability and cost of labour involved in deploying the traditional pheromone dispensers, which are devices that must be manually deployed in the field. As described here, we established a pipeline from Camelina engineering for pheromone fatty acid production, field cultivation of engineered lines, pheromone precursor fatty acid extraction and enrichment from engineered Camelina seed oil, pheromone synthesis from Camelina-derived fatty acids, and finally, effective insect pest monitoring and mating disruption using sustainably produced pheromone formulations that are flowable and thus amenable to field application using conventional farm equipment. Notably, we found that plant-derived insect pheromones are as effective as conventional synthetic pheromones for monitoring and mating disruption, regardless of the product purities. Hence, we demonstrated that engineered Camelina can be used as a plant factory for scalable production of moth sex pheromone precursors of high market value.
Insects have evolved conserved biosynthetic pathways employing a limited number of key enzymes controlling fatty acid synthesis, desaturation, limited chain shortening or elongation and the downstream reactions to modify the oxygen-containing functional groups30. In an engineered heterologous platform, it is possible to reconstruct the insect biosynthetic pathway or to design novel pathways that lead to the production of the same compound. Camelina, which has high yield potential and oil content and simple genetic transformation methodology19, provides an ideal platform for production of moth pheromones. Monounsaturated C18, C16 and C14 pheromone precursor acids with double bonds in ∆9 or ∆11 position can be produced by only slight modification of the available pool of saturated fatty acids. Our studies show the feasibility of introducing different thioesterases for export of optimal chain length fatty acids from the plastid and co-expression of desaturases with the desired substrate and product specificity to generate pheromone precursor fatty acids with limited impacts on plant fitness. Introducing desaturases with different fatty acid chain length and regiospecificities into a Camelina production platform could expand the portfolio of monounsaturated pheromone precursor acids. Production of pheromone compounds with shorter chain length or with multiple double bonds involves additional challenges introducing the necessary genes and controlling their action. Notably, the individual variation among T2 seeds was observed, but the selection during the following generation was aimed to take care of this, generating stable production of the target acids in the seed material. After selection over three generations of Camelina, the level of the target precursor Z11-16:Acid in the seed oil was stabilized around 20%. On the basis of variation in the target acid production between parental seeds multiplied in greenhouses and those harvested in the field, it is likely that careful selection of field production sites based on environmental conditions, including growth temperatures, will be useful for optimizing pheromone precursor fatty acid production levels.
Field data from the monitoring trap experiment demonstrated that the plant-derived pheromone lures are attractive to P. xylostella male moths, independent of their purity. We also found that traps with plant-derived pheromone lures were as efficient as traps with high-purity synthetic pheromone lures in attracting and trapping these moths. Traps with plant-derived pheromone lures, independent of their purity, captured moths that oriented upwind, located the pheromone point source and landed on it. Plant-derived pheromone, therefore, can be used in lures for monitoring traps (Fig. 5a) and in baits for attract-and-kill formulations containing minute amounts of pesticide to kill attracted insects15. The mating disruption trial, using trap shutdown as an indication of suppression of orientation of males to mates, also demonstrated that the plant-derived pheromone mating disruption formulations were as effective as the high-purity synthetic pheromone mating disruption formulation. Because plant-derived pheromone formulations disrupted the males’ ability to locate sex pheromone sources in the field, a key behavioural mechanism underlying mating disruption12, it is conceivable that these plant-derived pheromones will be useful in the establishment of commercial pest population suppression through mating disruption. It is much more difficult to induce impairment of source finding in small test plots than larger treated areas; so our data also suggest that mating disruption in areawide programmes might be achieved with lower doses of pheromone per treated area. Together, the results of these field trials suggest that Camelina-derived pheromone could effectively replace petroleum-based synthetic pheromone when formulating pest management products.
Conventional pheromone synthesis requires complex dedicated manufacturing infrastructures. Conventional pheromone synthesis is frequently a long and complex process, involving the import and transport of raw materials, chemical modification using large amounts of organic solvents and expensive catalysts and usually, production of toxic by-products, which are expensive and difficult to dispose of. Furthermore, this long process requires large financial investments over many months to obtain the target AIs for formulation and commercial use. All of these challenges combine to put pheromone-based control methods beyond the financial reach of growers of anything but high-value crops, such as orchard fruit and vegetables, with most pheromone AIs costing between US$1,000–3,500 kg−1 (ref. 15). Usually, pheromone formulations developed for mating disruption require 40–120 g ha−1 of AI15. In pheromone AI alone, formulators will spend from US$40 ha−1 for the lowest dose of the most inexpensive pheromones, up to US$400 ha−1 for the highest dose of the most expensive pheromones. Pheromone AI usually accounts for 70% of the cost of the formulation and ~30% of the cost of the final product to the grower. As a result, the area covered by crop protectants using sex pheromones as AIs has been relegated almost exclusively to high-value crops over the past five decades, reaching ~1 million ha globally13, which is a very small fraction of the ~1.5 billion ha of total cultivated area (Food and Agriculture Organization of the United Nations: https://www.fao.org/sustainability/news/detail/en/c/1274219).
New bio-based methods of pheromone production have the potential to reduce the cost of these AIs. In pheromone manufacturing, production of Z11-16:Acid using a conventional synthetic chemistry path is estimated to cost US$150–US$400 kg−1, depending on the pathway, whereas bioproduction of Z11-16:Acid through the Camelina pathway costs between US$10 kg−1 and US$25 kg−1, depending on the agronomical production of the crop and industrial extraction and isolation protocols utilized (A.M.-N., unpublished observations). The transformation of the acid precursor into the final sex pheromone adds another US$60 kg−1 to US$100 kg−1 to the cost, depending on the final AI product. Bioproduction of pheromones also reduces the dependence of pheromone manufacturers on supply chains of petroleum-based raw materials (A.M.-N., unpublished observations). The remarkable high-frequency occurrence of low-probability events have substantial negative impact on global production and commerce over the past two decades and increased the uncertainty and complexity of global supply chains to unprecedented levels31 (McKinsey.com: https://www.mckinsey.com/business-functions/risk/our-insights/covid-19-implications-for-business and https://www.mckinsey.com/business-functions/operations/our-insights/why-now-is-the-time-to-stress-test-your-industrial-supply-chain).
Here we developed plants that accumulate sex pheromone precursors for downstream processing. A different approach would be to design genetically modified plants to release the volatiles in the field for the purpose of attraction or mating disruption in a push-and-pull approach32, but this strategy has not yet been proven to work in the field. For example, a wheat variety was stably transformed to release (E)-β-farnesene, the alarm pheromone of many aphid pests, but the technology has not yet demonstrated to suppress or control aphid populations in the field33. Xia et al34. transiently modified the tobacco, Nicotiana benthamiana, to release a mixture of noctuid moth pheromone components, more specifically Z11-16:OH, Z11-16:OAc and Z11-16:Ald, in the laboratory, but the engineering of plants to release specific moth pheromone blends at efficacious ratios and biologically relevant doses in the field for successful mating disruption remains an outstanding challenge34. In contrast, pheromones derived from downstream processing of precursors from oil crops could easily replace conventionally produced synthetic pheromone used by formulators for pest control tools used in mating disruption and trapping programmes. We anticipate initially growing up to 4,000 acres (1,618 ha) of engineered Camelina under a Plant Made Pharmaceutical and Industrial permit, obtained in consultation with United States Department of Agriculture Animal and Plant Health Inspection Service (USDA APHIS). This will provide the opportunity to optimize field and bioprocessing production and logistic methods at commercial scale. Given that the engineered Camelina may produce 150 kg to 350 kg of precursor per hectare, this production level would yield ≥566 metric tons of precursor or enough to generate mating disruption formulation for ≥14.5 million acres of row crops. Plots under USDA APHIS permit are considered experimental production and require a high degree of grower stewardship to be compliant with permit conditions. Because of the cost and risks associated with permit production, we may consider deregulation of these lines, a process that may cost > US$3 million and take several years. Deregulation of these lines, which should streamline production, will ultimately be an economic decision that balances market demands, cost and risks.
Genetically modified Camelina, engineered to produce insect pheromone precursors, can pave the way for adoption of pheromone-based control methods in lower-value but higher-volume row crops, such as maize, cotton and soybean, where pheromone-based solutions currently have very little penetration. Efficient downstream processing and scale up of the production of sex pheromone products, based on pheromone precursors produced by genetically modified Camelina seeds, is essential to achieve the economic threshold needed for the adoption of pheromone tools in row crop pest protection. Costs of biological production relative to conventionally produced pheromones will depend on the purities needed for successful application. Whereas attraction of male moths to traps may depend on high purity and specific ratios of pheromone components, it is likely that successful mating disruption may be achieved with lower purity AIs. We estimate that US$30 ha−1 per application is the upper threshold cost to the grower for the adoption of pheromone-based pest control solutions in row crops, which may be achieved if the cost of AI production can be reduced to US$100 kg−1 or less. This would allow pheromone-based pest control products to finally compete with conventional pesticides, reducing global reliance on these environmentally hazardous chemicals. Additional positive impacts of bio-based pheromones include increased capacity to control pests that have developed resistance to traditional pesticides, promotion of integrated pest management and organic farming, production of agricultural products with no insecticide residues and improved crop protection due to high specificity of pheromones, preserving non-target species and preventing secondary pest problems.
Methods
Generation of transgene constructs and transformation
Camelina (Camelina sativa) var. Suneson, previously engineered for seed-specific expression of a Cuphea FatB thioesterase, was used as the transformation background22,23. Plants were grown on a 14 h day/10 h night, with day lengths maintained as needed with supplemental lighting (400–500 μmoles m−2 s−1) in a greenhouse. Day temperatures were maintained at ~25 °C, and night temperatures were held at ~19 °C.
The pheromone gland-specific fatty acyl-CoA desaturase Atr∆11 cDNA from the navel orangeworm, Amyelois transitella, was introduced into the binary vector pBinGlyBar135 using Gateway cloning. The Atr∆11 cDNA was flanked on its 5′ end by the soybean glycinin-1 promoter and on its 3′ end by the soybean glycinin-1 3′UTR. The pBinGlyBar1 binary vector contains a constitutively expressed resistance gene for the herbicide phosphinothricin (or Basta). The resulting binary vector was introduced into Agrobacterium tumefaciens C58.
A. tumefaciens cells harbouring the binary vector were transformed by floral infiltration into the high palmitic acid Camelina background as previously described36. Engineered lines from collected T1 seeds were screened for Basta resistance as described35. This selection identified >18 independent T1 lines. T2 seeds from these lines were screened for (Z)-11-hexadecenoic acid production by gas chromatography-flame ionization detection as described below. Top (Z)-11-hexadecenoic acid-producing lines were advanced under greenhouse conditions to homozygosity in the T3 generation.
Characterization of positively transformed plants
The seeds harvested from the florally dipped plants were sown in the soil for Basta selection. The surviving T1 plants were considered potential positive transformants and were grown until fully mature. Seeds produced from the T1 plants were sampled for chemical analysis to confirm the transformation.
Fatty acid composition analysis
Fatty acid methyl esters (FAMEs) were generated either by grinding pooled 25 individual seeds (for production analysis of one transformant) or by grinding each of 15 individual seeds per plant separately (for variation analysis within one transformant) in 1 ml 2% H2SO4 in methanol for 1 h at 90 °C in a 4 ml glass vial. After cooling, 1 ml water and 1 ml heptane were added and vortexed. Then, the heptane phase containing the FAMEs was transferred to 1.5 ml autosampler vials for chemical analysis.
An Agilent 5975 mass-selective detector coupled to an Agilent 6890 series gas chromatograph (GC/MS) equipped with a polar column (HP-INNOWax, 30 m length × 0.25 mm ID, 0.25 μm film thickness) was used for seed fatty acid composition analysis. Helium was used as carrier gas, and samples were injected under a splitless mode, with a constant flow of 1 ml min−1 corresponding to a linear velocity of 36 cm s−1. The inlet temperature was set at 260 °C, and the oven temperature was set at 80 °C for 1 min, then increased to 230 °C at a rate of 10 °C min−1 and held for 10 min. The temperature of transfer line and MS source were set at 280 °C and 230 °C, respectively. Reference synthetic fatty acid methyl esters (Supelco 37 FAME mix, Sigma-Aldrich, or individual compound from our lab stock) were used to confirm the identity of each fatty acid component in the seed lipid extracts in the form of their methyl esters, by comparing their retention times and mass spectra obtained from both polar and non-polar columns. Dimethyl disulfide derivatization was performed with the samples of seed FAMEs to confirm the double bond position in the target compound37. The samples of Dimethyl disulfide-adducts were analysed by an Agilent 5975 mass-selective detector coupled to an Agilent 7890 series gas chromatograph equipped with a non-polar column (HP-5MS, 30 m × 0.25 mm, 0.25 μm), for which the oven temperature was programmed at 80 °C for 2 min, then increased at a rate of 15 °C min−1 to 140 °C, and then increased at a rate of 5 °C min−1 to 260 °C and held for 30 min.
Establishment and execution of field trials
Target compound production in the selected CpuFatB + AtrD11 transgenic line was evaluated under field conditions in Borgeby, Skåne (55° 75′ 22.0″ N, 13° 05′ 01.0″ E, 973 m2, 30 May to 29 September, 2017), (55° 75′ 17.8″ N, 13° 05′ 01.9″ E, 1,375 m2, 11 June to 31 August, 4 September and 2 November, 2018), (55° 75′ 17.6″ N, 13° 04′ 99.6″ E, 1,440 m2, 13 May to 26 August and 19 September, 2019) with permission from the Swedish Board of Agriculture (Dnr 4.6.18-567/17) and near Ithaca, Nebraska (41° 08′ 47.4″ N, 96° 26′ 17.0″ W). In Nebraska, the engineered lines were grown in plots in a dedicated biotechnology field at the Eastern Nebraska Research and Extension Center (ENREC) in Mead, Nebraska, with plantings in late-March/mid-April of 2016 and 2018 and harvest in mid-July 2016 and 2018. Field trials were conducted under a permit from USDA APHIS.
Seed oil process, target compound extraction and purification
Approximately 0.3 kg and 4.3 kg (including 1.8 kg from Nebraska and 2.5 kg from Skåne) seed material, harvested from field trials in 2016 and 2018, respectively, were extracted in batches at room temperature. The seeds were ground and immersed in 2 volumes of n-heptane in a 2.5 l flask and stirred overnight. After extraction, the organic phase was filtered through a Buchner funnel, and the solvent was removed by a rotary evaporator. The fatty acid content in the resulting seed oil was transformed into corresponding methyl esters by base methanolysis in a 2 l flask. For each batch of the crude oil, 2 volumes of 0.5 M KOH in methanol was added and the mixture was stirred overnight at room temperature. The reaction was monitored by TLC until the band of triacylglycerol disappeared, and then an equal volume of 0.5 M HCl was added, and a volume of n-heptane equal to the neutralized mixture was added to extract the FAME product. The heptane phase containing the FAMEs was collected by a separatory funnel and rinsed twice with water, and finally, the organic solvent was recycled and water removed by a rotary evaporator under reduced pressure and at 65 °C for 1 h.
The C16 FAME components containing the target compound were isolated from the methanolysis products via short-path distillation (Kugelrohr, Aldrich). For that, ~60 g, 350 g and 490 g of FAME products extracted from 0.3 kg, 1.8 kg and 2.5 kg seeds were distilled under high vacuum (Edwards Advanced Vacuum) and at temperatures ramping from 125 °C to 140 °C, with a gradient of 5 °C. The distillates collected at the same temperature were combined and checked by GC/MS before undergoing further processing.
To remove the saturated component from the short-path distillates, urea complexation was performed following the Hayes et al. protocol with some modifications24. On a scale of 300 ml methanol, 40 g of FAME distillate was added and the solution allowed to achieve homogeneity at 65 ± 1 °C. While solution temperature was maintained, 63 g (7 mol equivalent) of urea was added. Once the urea was totally dissolved, the solution was rapidly cooled down to 18–20 °C by shaking the reaction flask under cold tap water. The resultant slurry was then gravity filtrated, and the complexes were separated from the filtrate. Recovered complexes were washed with isooctane, a non-complexing solvent, to remove traces of the filtrate. Warm water (70 °C, 400 ml) and a small volume (10 ml) of n-heptane were applied to recover FAMEs from both filtrate in methanol and isolated complexes. The resulting FAME samples from top phase of filtrates and decomposed complexes were checked on GC/MS. Depending on the composition of saturated and unsaturated C16 acids in the starting mixture, one or two rounds of complexation were performed to obtain the purified target acid in the filtrate.
Additional silver nitrate (AgNO3)-silica gel column chromatography was applied to the isolated FAME samples obtained from seeds of the 2016 field trial. After short-path distillation and urea complexation, the isolated samples containing mostly C16 components were loaded on an AgNO3-silica gel-based column (AgNO3 5%) and eluted by n-heptane:diethyl ether:acetic acid (85:15:1 by volume). Fractions with the highest percentage of Z11-16 component were pooled (having 38% of Z11-16 methyl ester), and the majority of the pooled fractions was used in the synthesis of the final pheromones. A minor part of the pooled fraction was further separated on AgNO3-impregnated silica TLC plates (Silica 60, Merck) developed in n-heptane:diethyl ether:acetic acid (85:15:1 by volume). The monounsaturated band (containing 83% Z11-16 methyl ester) was eluted from the gel and used for synthesis of the final pheromones.
Chemical transformation of precursor acid to pheromone compounds
The purified precursor acid in the form of its methyl ester was converted into the corresponding alcohol using lithium aluminium hydride (LiAlH4) (conversion rate 98%, yield 88%). The fatty alcohol product was oxidized into aldehyde using pyridinium chlorochromate according to Corey and Suggs38 or acetylated into acetate pheromone by Fischer esterification with acetyl chloride nearly quantitatively.
Monitoring of the diamondback moth, Plutella xylostella
In the field trial (8 March to 3 May, 2017), four treatments including a blank control (N = 5), an optimal synthetic pheromone mixture of aldehyde, acetate and alcohol in a blend ratio of 30:70:0.1, and two seed oil-derived pheromone mixtures with different purities but with the AIs in the same amounts and proportions (as confirmed by GC analysis) were evaluated in an experimental field of Choy sum, Brassica rapa var. parachinensis in Guangzhou, China (22° 48′ 14.0″ N, 113° 26′ 26.0″ E, 54,000 m2). The synthetic pheromone compounds with a purity of ≥99% were available from our laboratory collection. For each treatment, there were five replicates, and during the experimental period, traps (Csalomon, Budapest, Hungary) were replaced once with fresh pheromone baits by the end of week 6. The dispensers (red rubber septa, catalogue number 224100-020, Wheaton Science Products) were loaded with 100 µg AI per septum dissolved in 100 µl heptane and stored at −20 °C before use in the field. To install the bait in a trap, a pin was used to pierce the rubber septum through the sidewall of the trap.
Traps within a replicate were randomly placed in a row in the field on sticks at ~1 m height above ground level, separated from each other by 10–15 m. The traps were checked once per week. After each check, the traps were redistributed successively within a replicate row to eliminate any potential position effect.
Field experiment for trap shutdown
A trap shutdown experiment was performed in a field of common dry beans, Phaseolus vulgaris, in Fazenda Van den Broek, Parapanema, São Paulo, Brazil (23° 27′ 37.5″ S 48° 48′ 29.3″ W), targeting the noctuid species, H. armigera, a global agricultural pest that uses Z11-16:Ald as its major sex pheromone component, together with a minor component (Z)-9-hexadecenal (Z9-16:Ald)28,39. Trap catches were monitored in 30 m × 30 m plots using two Delta traps per plot placed 20 cm above the crop and baited with commercial H. armigera pheromone rubber septum lures (ISCAlure Armigera, ISCA Inc. USA). The experiment included three different treatments, each containing 1.8 g of the AI (Z11-16:Ald) per plot, two of them using plant-derived pheromone with a purity of 45% and 75%, respectively (Supplementary Fig. 4) and the third using conventionally produced synthetic pheromone, with a purity of ≥98% (ISCA Inc. USA). Untreated plots receiving no pheromone mating disruption treatment served as a negative control. Number of replicates was four, each replicate with two monitoring traps checked weekly. Monitoring of the experimental plots before the installation of the mating disruption experiment indicated the presence of H. armigera at similar population densities across all fields.
Pheromone application to treatment plots in a SPLAT formulation was performed with a mechanical applicator. The splat portions were 0.5 g totalling 2,000 portions per ha. Thus, in each plot, 90 portions of 0.5 g were applied in six strips spaced 5 m apart. In each strip, 15 portions were applied 2 m apart. The different treatment plots were 50 m apart. Monitoring traps were placed in the middle of the plot between strips 2 and 3 and 4 and 5, respectively.
Statistical analysis
The statistical analysis of trap catches (one-way analysis of variance (ANOVA) followed by a least significant difference (LSD) or Tukeyʼs test at P < 0.05 level) was performed with IBM SPSS Statistics 26.0.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Sallam, M. N. in Insect Damage: Post-Harvest Operations (ed Mejia, D.) 1–38 (AGSI/FAO, 2000).
Deutsch, C. et al. Increase in crop losses to insect pests in a warming climate. Science 361, 916–919 (2018).
Sánchez-Bayo, F. in Encyclopedia of the Anthropocene (eds Dellasala, D. A. & Goldstein, M. I.) 111–117 (Elsevier, 2018).
Buszewski, B., Bukowska, M., Ligor, M. & Staneczko-Baranowska, I. A holistic study of neonicotinoids neuroactive insecticides—properties, applications, occurrence, and analysis. Environ. Sci. Pollut. Res. 26, 34723–34740 (2019).
Sharma, A. et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 1, 1146 (2019).
Cardé, R. T. in Perspectives in Ecological Theory and Integrated Pest Management (ed Kogan, M.) 122–169 (Cambridge Univ. Press, 2007).
Gut, L. J., Stelinski, L. L., Thomson, D. R. & Miller, J. R. in Integrated Pest Management: Potential, Constraints, and Challenges (eds Koul, O. et al.) 73–120 (CABI Publishing, 2004).
Suckling, D. M. in Pheromone Communication in Moths (eds Allison, J. D. & Cardé, R. T.) 337–347 (Univ. of California Press, 2016).
Cork, A. in Pheromone Communication in Moths (eds Allison, J. D. & Cardé, R. T.) 349–363 (Univ. of California Press, 2016).
Evenden, M. in Pheromone Communication in Moths (eds Allison, J. D. & Cardé, R. T.) 365–393 (Univ. of California Press, 2016).
Wyatt T. D. (ed) Pheromones and Animal Behavior: Chemical Signals and Signatures 2nd edn (Cambridge Univ. Press, 2014).
Miller, J. R. & Gut, L. J. Mating disruption for the 21st century: matching technology with mechanism. Environ. Entomol. 44, 427–453 (2015).
Witzgall, P., Kirsh, P. A. & Cork, A. Sex pheromones and their impact on pest management. J. Chem. Ecol. 36, 80–100 (2010).
Holkenbrink, C. et al. Production of moth sex pheromones for pest control by yeast fermentation. Metab. Eng. 62, 312–321 (2020).
Mafra-Neto, A. et al. in Natural Products for Pest Management (eds Beck, J. et al.) Ch. 4 (American Chemical Society, 2013).
Hagström, Å. K. et al. A moth pheromone brewery: production of (Z)-11-hexadecenol by heterologous co-expression of two biosynthetic genes from a noctuid moth in a yeast cell factory. Microb. Cell Fact. 12, 125 (2013).
Ding, B. J. et al. A plant factory for moth pheromone production. Nat. Commun. 5, 3353 (2014).
Xia, Y. H. et al. Production of moth sex pheromone precursors in Nicotiana spp.: a worthwhile new approach to pest control. J. Pest Sci. 93, 1333–1346 (2020).
Eynck, C. & Falk, K. C. in Fuel Crops: Production, Physiology and Genetics (ed Singh, B.P.) 369–391 (CAB International, 2013).
Iskandarov, U., Kim, H. J., & Cahoon, E. B. in Plants and BioEnergy (eds McCann, M. C.et al.) 131–140 (Springer, 2014).
Han, L. et al. High level accumulation of EPA and DHA in field-grown transgenic Camelina—a multi-territory evaluation of TAG accumulation and heterogeneity. Plant Biotechnol. J. 18, 2280–2291 (2020).
Kim, H. J. et al. Toward production of jet fuel functionality in oilseeds: identification of FatB acyl-acyl carrier protein thioesterases and evaluation of combinatorial expression strategies in Camelina seeds. J. Exp. Bot. 66, 4251–4265 (2015).
Horn, P. J. et al. Imaging heterogeneity of membrane and storage lipids in transgenic Camelina sativa seeds with altered fatty acid profiles. Plant J. 76, 138–150 (2013).
Hayes, D. G., Bengtsson, Y. C., Van Alstine, J. M. & Setterwall, F. Urea complexation for the rapid, ecologically responsible fractionation of fatty acids from seed oil. J. Am. Oil Chem. Soc. 75, 1403–1409 (1998).
Chow, Y. S., Lin, Y. M. & Hsu, C.-L. Sex pheromone of the diamondback moth (Lepidoptera: Plutellidae). Bull. Inst. Zool. Acad. Sin. 16, 99–105 (1977).
Tamaki, Y. et al. (Z)-11-hexadecenal and (Z)-11-hexadecenyl acetates: sex-pheromone components of the diamondback moth (Lepidoptera: Plutellidae). Appl. Entomol. Zool. 12, 208–210 (1977).
Dai, J. Q. et al. Development of regional attractants for diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) based on sex pheromones and its application in Pearl River Delta. J. Environ. Entomol. 38, 1204–1209 (2016).
Nesbitt, B. F., Beevor, P. S., Hall, D. R. & Lester, R. Female sex pheromone components of the cotton bollworm, Heliothis armigera. J. Insect Physiol. 25, 535–541 (1979).
Faleiro, J. R. et al. Controlled release products for managing insect pests. Outlooks Pest Manag. 27, 175–180 (2016).
Blomquist, G. J., Jurenka, R., Schal, C. & Tittiger, C. in Insect Endocrinology (ed Gilbert, L. I.) 523–567 (Academic Press-Elsevier, 2012).
Choudhary, N.A., Singh, S., Schoenherr, T. & Ramkumar, M. Risk assessment in supply chains: a state-of-the-art review of methodologies and their applications. Ann. Oper. Res. https://doi.org/10.1007/s10479-022-04700-9 (2022).
Cook, S. M., Khan, Z. R. & Pickett, J. A. The use of push–pull strategies in integrated pest management. Annu. Rev. Entomol. 52, 375–400 (2007).
Bruce, T. et al. The first crop plant genetically engineered to release an insect pheromone for defence. Sci. Rep. 5, 11183 (2015).
Xia, Y. H. et al. Release of moth pheromone compounds from Nicotiana benthamiana upon transient expression of heterologous biosynthetic genes. BMC Biol. 20, 80 (2022).
Nguyen, H. T. et al. Camelina seed transcriptome: a tool for meal and oil improvement and translational research. Plant Biotechn. J. 11, 759–769 (2013).
Lu, C. & Kang, J. Generation of transgenic plants of a potential oilseed crop Camelina sativa by Agrobacterium-mediated transformation. Plant Cell Rep. 27, 273–278 (2008).
Dunkelblum, E., Tan, S. H. & Silk, P. J. Double-bond location in monounsaturated fatty acids by dimethyl disulfide derivatization and mass spectrometry: application to analysis of fatty acids in pheromone glands of four Lepidoptera. J. Chem. Ecol. 11, 265–277 (1985).
Corey, E. J. & Suggs, J. W. Pyridinium chlorochromate. An efficient reagent for oxidation of primary and secondary alcohols to carbonyl compounds. Tetrahedron Lett. 16, 2647–2650 (1975).
Nesbitt, B. F., Beevor, P. S., Hall, D. R. & Lester, R. (Z)-9-hexadecenal: a minor component of the female sex pheromone of Heliothis armigera (Hübner) (Lepidoptera, Noctuidae). Entomol. Exp. Appl. 27, 306–308 (1980).
Acknowledgements
This work was supported by funding from the Swedish Foundation for Strategic Research (number RBP 14-0037, Oil Crops for the Future) to S.S. and C.L., Formas (number 2010-857 and 2015-1336) to C.L. and from the USDA National Institute of Food and Agriculture, Small Business Innovation Research (SBIR number 2020-33610-32836) to A.M.-N. Additionally, E.B.C. recognizes support from Nebraska Agricultural Experiment Station-USDA Hatch Act (NEB-30-131), and P.H. recognizes support from the strategic research programme Trees and Crops for the Future (TC4F). We thank Hushållningssällskapet Skåne and colleagues in the Lund University pheromone group for their assistance in the field trial in Sweden and C. Bernardi, R.O. da Silva and R. Lake from ISCA Inc. USA for their assistance in characterizing and formulating the materials for the trap shutdown field experiment in São Paulo, Brazil.
Funding
Open access funding provided by Lund University
Author information
Authors and Affiliations
Contributions
S.S., P.H., E.B.C. and C.L. conceived the study. P.H. and B.-J.D. carried out vector design and construction. E.B.C. and T.J.N. performed floral dip transformation, plant cultivation, sample analysis and field propagation in Nebraska, USA. H.-L.W., B.-J.D., C.L. and P.H. performed plant breeding and field propagation in Lund, Alnarp and Borgeby, Sweden. S.S. and H.-L.W. performed seed oil processing, fatty acid isolation and chemical conversion. J.-Q.D. performed monitoring experiments in Guangdong, China. R.B. and A.M.-N. performed the trap shutdown experiment in São Paulo, Brazil. H.-L.W. and C.L. drafted the manuscript. All authors edited the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Ethics declaration
The Swedish Board of Agriculture (Jordbruksverket) approved the protocol of studying the genetically modified seeds in the lab. Field trials were conducted under a permit from the Swedish Board of Agriculture (Dnr 4.6.18-567/17) and the USDA APHIS.
Competing interests
A.M.-N. is the CEO and shareholder of ISCA Inc., Riverside, CA, USA, with interests in the technology of the subject matter. C.L. and P.H. are founders of the company SemioPlant AB with interests in the technology of the subject matter. B.-J.D., C.L., E.B.C., H.-L.W., P.H. and S.S. are inventors on or have financial interests in patent applications related to insect pheromone production in plants (WO 2015/171057 A1, US 11162111 B2, EP 3167070 B1). The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Lukasz Stelinski, Junwei Zhu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–4.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, HL., Ding, BJ., Dai, JQ. et al. Insect pest management with sex pheromone precursors from engineered oilseed plants. Nat Sustain 5, 981–990 (2022). https://doi.org/10.1038/s41893-022-00949-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-022-00949-x
This article is cited by
-
Effect of different bagging materials on guava fruit physiology and its quality attributes
Plant Physiology Reports (2023)
-
Something in the air
Nature Sustainability (2022)