Abstract
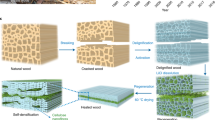
Synthetic fibres such as polyester and carbon are used in a broad variety of industries. However, as they derive from petrochemicals that are neither renewable nor biodegradable, the development of natural alternatives has gained increasing momentum in recent years. Here, we report a top-down approach for scalable production of cellulose macrofibres from bamboo stems involving a mild delignification process followed by water-assisted air-drying. Consisting of aligned and densely packed cellulose nanofibrils that possess strong hydrogen bonds and van der Walls forces, the extracted fibres exhibit a tensile strength of 1.90 ± 0.32 GPa, a Young’s modulus of 91.3 ± 29.7 GPa and a toughness of 25.4 ± 4.5 MJ m−3, which exceed those of wood-derived fibres and are comparable to synthetic carbon analogues. As a result of the low density, the specific strength is as high as 1.26 ± 0.21 GPa cm−3 g−1, surpassing most reinforcing components such as steel wire, synthetic polymers and vitreous fibres. The life-cycle assessment reveals that replacing polymer and carbon fibres in structural composites with the current natural fibres leads to substantial reduction in carbon emissions. Our work suggests a pathway towards sustainability in wider areas of application, including automobiles, aeronautics and construction.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data are available upon reasonable request from the authors, according to their contributions.
References
Chae, H. G. & Kumar, S. Making strong fibers. Science 319, 908–909 (2008).
Kim, D. J., Park, S. H., Ryu, G. S. & Koh, K. T. Comparative flexural behavior of hybrid ultra high performance fiber reinforced concrete with different macro fibers. Constr. Build. Mater. 25, 4144–4155 (2011).
Ellringmann, T., Wilms, C., Warnecke, M., Seide, G. & Gries, T. Carbon fiber production costing: a modular approach. Text. Res. J. 86, 178–190 (2016).
Li, K. et al. Alignment of cellulose nanofibers: harnessing nanoscale properties to macroscale benefits. ACS Nano 15, 3646–3673 (2021).
Mittal, N. et al. Multiscale control of nanocellulose assembly: transferring remarkable nanoscale fibril mechanics to macroscale fibers. ACS Nano 12, 6378–6388 (2018).
Zhang, H. et al. Regenerated-cellulose/multiwalled-carbon-nanotube composite fibers with enhanced mechanical properties prepared with the ionic liquid 1-allyl-3-methylimidazolium chloride. Adv. Mater. 19, 698–704 (2007).
Li, Y. et al. Hybridizing wood cellulose and graphene oxide toward high-performance fibers. NPG Asia Mater. 7, e150 (2015).
Lee, W. J., Clancy, A. J., Kontturi, E., Bismarck, A. & Shaffer, M. S. Strong and stiff: high-performance cellulose nanocrystal/poly (vinyl alcohol) composite fibers. ACS Appl. Mater. Inter. 8, 31500–31504 (2016).
Li, T. et al. Developing fibrillated cellulose as a sustainable technological material. Nature 590, 47–56 (2021).
Jia, C. et al. From wood to textiles: top-down assembly of aligned cellulose nanofibers. Adv. Mater. 30, 1801347 (2018).
Ku, H., Wang, H. & Pattarachaiyakoop, N. A review on the tensile properties of natural fiber reinforced polymer composites. Compos. B 42, 856–873 (2011).
Habibi, M. K. & Lu, Y. Crack propagation in bamboo’s hierarchical cellular structure. Sci. Rep. 4, 5598 (2014).
Yu, Y., Jiang, Z., Fei, B., Wang, G. & Wang, H. An improved microtensile technique for mechanical characterization of short plant fibers: a case study on bamboo fibers. J. Mater. Sci. 46, 739–746 (2011).
Zakikhani, P. et al. Extraction and preparation of bamboo fibre-reinforced composites. Mater. Des. 63, 820–828 (2014).
Osorio, L., Trujillo, E., Van, Vuure, W. & Verpoest, I. Morphological aspects and mechanical properties of single bamboo fibers and flexural characterization of bamboo/epoxy composites. J. Reinf. Plast. Comp. 30, 396–408 (2011).
Deshpande, A., Bhaskar, M. & Lakshmana, C. Extraction of bamboo fibers and their use as reinforcement in polymeric composites. J. Appl. Polym. Sci. 76, 83–92 (2000).
Terashima, N., Fukushima, K., He, L. F. & Takabe, K. in Forage Cell Wall Structure and Digestibility (eds Jung, H. G. et al.) 247–270 (ASA, CSSA, and SSSA Books, 1993).
Brännvall, E. The limits of delignification in kraft cooking. BioResources 12, 2081–2107 (2017).
Perez, D. D. S. et al. Peroxyformic acid pulping of Eucalyptus grandis wood chips and sugar cane bagasse in one stage and characterization of the isolated lignins. J. Wood Chem. Technol. 18, 333–365 (1998).
Sun, R., Tomkinson, J., Geng, Z. & Wang, N. Comparative studies of hemicelluloses solubilized during the treatments of maize stems with peroxymonosulfuric acid, peroxyformic acid, peracetic acid, and hydrogen peroxide. Part 2. Spectroscopic and thermal characterizations. Holzforschung 54, 492–496 (2000).
Peng, Y., Gardner, D. J. & Han, Y. Drying cellulose nanofibrils: in search of a suitable method. Cellulose 19, 91–102 (2012).
Iyer, K. K., Neelakantan, P. & Radhakrishnan, T. Birefringence of native cellulosic fibers. I. Untreated cotton and ramie. J. Polym. Sci. Pol. Phys. 6, 1747–1758 (1968).
Wang, F. & Shao, Z. Study on the variation law of bamboo fibers’ tensile properties and the organization structure on the radial direction of bamboo stem. Ind. Crop. Prod. 152, 112521 (2020).
Wang, X., Ren, H., Zhang, B., Fei, B. & Burgert, I. Cell wall structure and formation of maturing fibres of moso bamboo (Phyllostachys pubescens) increase buckling resistance. J. R. Soc. Interface 9, 988–996 (2012).
Nishiyama, Y., Langan, P. & Chanzy, H. Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc. 124, 9074–9082 (2002).
Yoshiharu, N., Shigenori, K., Masahisa, W. & Takeshi, O. Cellulose microcrystal film of high uniaxial orientation. Macromolecules 30, 6395–6397 (1997).
Youssefian, S. & Rahbar, N. Molecular origin of strength and stiffness in bamboo fibrils. Sci. Rep. 5, 11116 (2015).
Xu, Z. et al. Ultrastiff and strong graphene fibers via full–scale synergetic defect engineering. Adv. Mater. 28, 6449–6456 (2016).
Wang, F. & Shao, J. Modified Weibull distribution for analyzing the tensile strength of bamboo fibers. Polymers 6, 3005–3018 (2014).
Trujillo, E. et al. Bamboo fibres for reinforcement in composite materials: strength Weibull analysis. Compos. A 61, 115–125 (2014).
Cambridge Engineering Selector Edupack Software (Granta Design Limited, 2017).
Wang, S. et al. Super–strong, super–stiff macrofibers with aligned, long bacterial cellulose nanofibers. Adv. Mater. 29, 1702498 (2017).
Shi, J., Fang, X., Maffe, A. P. & Yao, D. An effective method of processing immiscible polymer blends into strong fiber. Polym. Eng. Sci. 59, 2052–2061 (2019).
Chae, H. G. & Kumar, S. Rigid–rod polymeric fibers. J. Appl. Polym. Sci. 100, 791–802 (2006).
Deák, T. & Czigány, T. Chemical composition and mechanical properties of basalt and glass fibers: a comparison. Text. Res. J. 79, 645–651 (2009).
Xu, Z. et al. Ultrastrong fibers assembled from giant graphene oxide sheets. Adv. Mater. 25, 188–193 (2013).
Xin, G. et al. Highly thermally conductive and mechanically strong graphene fibers. Science 349, 1083–1087 (2015).
Zhang, X. et al. Ultrastrong, stiff, and lightweight carbon–nanotube fibers. Adv. Mater. 19, 4198–4201 (2007).
Bermudez, V. & Ogale, A. A. Adverse effect of mesophase pitch draw-down ratio on carbon fiber strength. Carbon 168, 328–336 (2020).
Sluiter, A. et al. Determination of Structural Carbohydrates and Lignin in Biomass, Laboratory Analytical Procedure (LAP) (NREL, 2008).
ASTM-D3800-99 — Standard Test Method for Density of High Modulus Fibers (American Society for Testing and Materials, 2010).
Nishiyama, Y., Langan, P. & Chanzy, H. Crystal structure and hydrogen bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc. 124, 9074–9082 (2002).
Turki, A., El Oudiani, A., Msahli, S. & Sakli, F. Investigation of OH bond energy for chemically treated alfa fibers. Carbohyd. Polym. 186, 226–235 (2018).
ISO 14040:2006 Environmental Management—Life Cycle Assessment—Principles and Framework (ISO, 2006).
ISO 14067:2018 Greenhouse Gases—Carbon Footprint of Products—Requirements and Guidelines for Quantification and Communication (ISO, 2018).
Xia, C., Shi, S. Q. & Cai, L. Vacuum-assisted resin infusion (VARI) and hot pressing for CaCO3 nanoparticle treated kenaf fiber reinforced composites. Compos. B 78, 138–143 (2015).
Acknowledgements
We acknowledge the support of the Maryland NanoCenter and its AIMLab, as well as the support from the University of Maryland A. James Clark School of Engineering.
Author information
Authors and Affiliations
Contributions
L.H., Z.L. and C.C. conceived the idea and designed the experiments. Z.L. contributed to the bamboo cellulose macrofibre extraction and characterization. Z.L., X.Z., R.B. and H.X. contributed to the analysis of the crystalline structure. J.L., H.X. and M.H. contributed to collection of the SEM and digital images. Y.Y. contributed to the cradle-to-gate life-cycle carbon analysis. S.Q.S. and L.M.S. contributed to the preparation of the composites. H.Q. provided characterization via FTIR and X-ray diffraction. X.P.Z. provided the Weibull statistics distribution of tensile strength. X.P. and Y.D. provided useful suggestions for chemical composition analysis. Z.L., C.C., A.B. and L.H. collaboratively analysed the data and wrote the manuscript. All authors commented on the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Sustainability thanks Zhaohui Tong and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 The cross-sectional photographs and SEM iamges of wood (basswood) and bamboo.
a, Cross-sectional photograph and b, SEM image of basswood, which features a cellular structure formed by numerous parallel hollow tube-like cells (for example, small tracheids and large vessels). c, Magnified SEM image showing the open vessels and tracheids, which exhibit a hollow structure with thin cell walls and large lumens (that is, the open space within the channel). d, Cross-sectional photograph and e, SEM image of natural bamboo. The material’s hollow parenchyma cells are arranged in a honeycomb pattern distributed around the solid macrofibers. f, Magnified SEM image showing the macrofibers, which consist of closely packed solid microfibrils.
Extended Data Fig. 2 The longitudinal-sectional SEM images of bamboo.
a, SEM image showing the side-view of the bamboo stem (cut parallel to the growth direction). b, Magnified SEM image of the hollow parenchyma cells, which are arranged in a honeycomb-like structure. c, Numerous pits distributed on the parenchyma cell walls provide water transport between channels. d, The long, rigid macrofibers composed of solid microfibrils provide longitudinal strength to the bamboo stem. e, The microfibrils are further composed of densely packed nanofibrils, which consist of dozens of directionally aligned cellulose molecular chains.
Extended Data Fig. 3 The chemical structure changes of lignin polymer during the delignification process.
a, Side-chain regions and b, aromatic regions in the HSQC NMR spectra of lignins extracted from natural bamboo stem. c, Side-chain regions and d, aromatic regions in the HSQC NMR spectra of lignins extracted from the partially delignified bamboo stem (after the delignification process for 3 hours). e, Side-chain regions and f, aromatic regions in the HSQC NMR spectra of lignins extracted from the partially delignified bamboo stem (after the delignification process for 5 hours).
Extended Data Fig. 4 The macroscopic structural changes of bamboo stem during the delignification process.
a, Photographs of the colour change in the bamboo surface, which indicates the chromophore groups (for example, methoxyl groups, conjugated double bonds, and syringyl units) of lignin have reacted with the peroxyformic acid during the delignification process. b, Photographs of the bamboo cross-section, showing the delignification treatment leads to the gradual separation of the bamboo into individual macrofibers, progressing from the inside to the outer regions of the stem over a period of several hours.
Extended Data Fig. 5 The 13C CP/MAS NMR spectra of natural bamboo stem and bamboo cellulose macrofibers.
13C CP/MAS NMR spectra of natural bamboo stem and bamboo cellulose macrofibers. Compared with natural bamboo stem, the disappeared signals at the regions of 152 - 110 and 56.0 ppm of bamboo cellulose macrofiber indicated that the aromatic ring structure and methoxyl groups of lignin were completely eliminated during the chemical delignification process. The absence of signals at 172.6 and 20.7 ppm as well as the decreased peak heights in the regions between 60 and 105 ppm suggested substantial hemicelluloses were removed, while the remained characteristic signals of cellulose suggested the natural cellulose molecular structure was well preserved during the chemical treatment.
Extended Data Fig. 6 The crystal structures and diameter distributions of mechanically extracted macrofibers and bamboo cellulose macrofibers.
a, A photograph of a typical mechanically extracted bamboo macrofiber (23 cm long) isolated from a manual peeling process without delignification treatment. b–d, As the isolated bamboo macrofiber was much longer than the field-of-view of the microscope, we observed this macrofiber from left to right along the longitudinal direction. The polarized optical microscopy images displayed a dark-coloured surface due to the presence of amorphous lignin and hemicelluloses. The scale bar is 100 μm. e, The mechanically extracted bamboo shows a non-uniform diameter of 190 – 236 μm along its length as partial parenchyma cell remained on the surface. f, A photograph of a typical bamboo cellulose macrofiber (23 cm long) isolated from the two-step delignification and drying process. g–i, The polarized optical microscopy images show strong birefringence at the different positions of the cellulose macrofiber, demonstrating that lignin has been uniformly removed from the cell walls and there is good alignment of the crystalline cellulose microfibrils. The scale bar is 100 μm. j, The bamboo cellulose macrofiber shows a uniform diameter of ~120 μm along its length, without any obvious defects.
Extended Data Fig. 7 Hydrogen bonding analysis of natural bamboo stem, mechanically extracted macrofibers and bamboo cellulose macrofibers.
a, The hydrogen bonds in the inter-chain and intra-chain structure of cellulose. b, The FTIR spectra and c, second derivative FTIR spectra of the natural bamboo stem, mechanically extracted macrofibers and bamboo cellulose macrofibers. Five band positions deriving from the second derivative spectra at 3270, 3340, 3433, 3560 and 3590 cm−1 are assigned to the O6-H⋯O3′ inter-molecular H-bond (peak 1), O3-H⋯O5 intra-molecular H-bond (peak 2), O2-H⋯O6 intra-molecular H-bond (peak 3), free O(2)H (peak 4) and free O(6)H (peak 5), respectively. d, e, f, The deconvoluted FTIR spectra of the d, bamboo cellulose macrofibers, e, mechanically extracted macrofibers, and f, natural bamboo stem.
Extended Data Fig. 8 Morphology observation of the mechanically extracted macrofibers and bamboo cellulose macrofibers.
a, A typical SEM image of the mechanically extracted macrofiber, which demonstrates a rough morphology and non-uniform diameter with a thick layer of parenchyma cells. b, The mechanically extracted macrofiber’s surface displays the presence of non-uniform fragments and voids. c, Magnified SEM image showing the detached nanofibrils on the surface of the microfibers the latter composes mechanically extracted macrofiber. d, A typical SEM image of a bamboo cellulose macrofiber obtained via the chemical delignification and air-drying process, which demonstrates a uniform diameter of ~180 μm, e, The bamboo cellulose macrofiber has a relatively smooth surface as most of the parenchyma cells have been removed. f, Highly aligned cellulose nanofibrils with a width of ~20–50 nm are visible on the microfibril surface of the bamboo cellulose macrofiber.
Extended Data Fig. 9 The fracture surfaces of the mechanically extracted macrofibers and bamboo cellulose macrofibers.
a, A photograph of a bamboo cellulose macrofiber after tensile testing. b, A SEM micrograph of the fracture morphology of a bamboo cellulose macrofiber. c, A magnified SEM micrograph shows the densely packed cellulose nanofibrils well-arranged along the longitudinal axis of bamboo cellulose macrofiber. d, A photograph of a mechanically extracted macrofiber after tensile testing. e A SEM micrograph of the fracture morphology of the mechanically extracted macrofiber. f, A magnified SEM micrograph shows the cellulose nanofibrils in the mechanically extracted macrofiber are still intertwined with lignin and hemicelluloses.
Extended Data Fig. 10 Twill fabric mat woven from bamboo cellulose macrofibers.
a, Approximately 1000 bamboo cellulose macrofibers were used to manually weave. b, A twill fabric mat with 0/90° aligned fibre orientation for the fabrication of an epoxy resin composite.
Supplementary information
Supplementary Information
Supplementary Notes 1–3, Figs. 1–17, Tables 1–7 and References 1–71.
Supplementary Video 1
Separation process showing the delignified bamboo stem was separated into thousands of distinct macrofibres.
Supplementary Video 2
The highly oriented bamboo cellulose macrofibres were well retained in water during the separation process.
Rights and permissions
About this article
Cite this article
Li, Z., Chen, C., Xie, H. et al. Sustainable high-strength macrofibres extracted from natural bamboo. Nat Sustain 5, 235–244 (2022). https://doi.org/10.1038/s41893-021-00831-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-021-00831-2
This article is cited by
-
Scalable production of carboxylated cellulose nanofibres using a green and recyclable solvent
Nature Sustainability (2024)
-
Power benefitted bioremediation of hexavalent chromium ions in biochar blended soil microbial fuel cell
Biomass Conversion and Biorefinery (2024)
-
Agave sisalana: towards distributed manufacturing of absorbent media for menstrual pads in semi-arid regions
Communications Engineering (2023)
-
Robust flexural performance and fracture behavior of TiO2 decorated densified bamboo as sustainable structural materials
Nature Communications (2023)
-
Comparative analysis of endophytic fungal communities in bamboo species Phyllostachys edulis, Bambusa rigida, and Pleioblastus amarus
Scientific Reports (2023)