Abstract
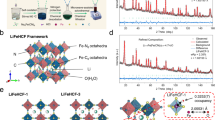
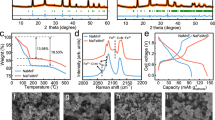
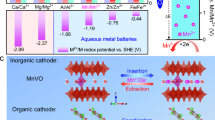
While lithium-ion batteries still dominate energy storage applications, aqueous potassium-ion batteries have emerged as a complementary technology due to their combined advantages in cost and safety. Realizing their full potential, however, is not without challenges. One is that among the limited choices of cathode materials, the more sustainable Prussian blue analogues suffer from fast capacity fading when manganese is present. Here we report a potassium manganese hexacyanoferrate K1.82Mn[Fe(CN)6]0.96·0.47H2O cathode featuring an in situ cation engineered surface where iron is substituted for manganese. With this engineered surface, the cathode design exhibits a discharge capacity of 160 mAh g−1 and 120 mAh g−1 at 300 mA g−1 and 2,500 mA g−1, respectively, and sustains 130,000 cycles (more than 500 days) with negligible capacity loss. Pairing the current cathode with a 3,4,9,10-perylenetetracarboxylic diimide anode yields a full potassium-ion cell that delivers an energy density as high as 92 Wh kg−1 and retains 82.5% of the initial capacity after 6,500 cycles at 1,500 mA g−1. The unprecedented electrochemical performance could be attributed to the suppressed manganese dissolution as a result of the shielding surface layer. This work may open an avenue for the rational design of high-performance cathode materials with redox-active manganese for rechargeable batteries.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the plots depicted in this manuscript and other findings of this study are available upon request from the corresponding author.
References
Zhang, W. C., Liu, Y. & Guo, Z. P. Approaching high-performance potassium-ion batteries via advanced design strategies and engineering. Sci. Adv. 5, eaav7412 (2019).
Yu, M. et al. Aqueous lithium-iodine solar flow battery for the simultaneous conversion and storage of solar energy. J. Am. Chem. Soc. 137, 8332–8335 (2015).
Liu, T. et al. Understanding Co roles towards developing Co-free Ni-rich cathodes for rechargeable batteries. Nat. Energy 6, 277–286 (2021).
Hosaka, T., Kubota, K., Hameed, A. S. & Komaba, S. Research development on K-ion batteries. Chem. Rev. 120, 6358–6466 (2020).
Xiao, N., McCulloch, W. D. & Wu, Y. Y. Reversible dendrite-free potassium plating and stripping electrochemistry for potassium secondary batteries. J. Am. Chem. Soc. 139, 9475–9478 (2017).
Liu, Y. et al. Boosting potassium-ion batteries by few-layered composite anodes prepared via solution-triggered one-step shear exfoliation. Nat. Commun. 9, 3645 (2018).
Yang, C. et al. Aqueous Li-ion battery enabled by halogen conversion-intercalation chemistry in graphite. Nature 569, 245–250 (2019).
Suo, L. et al. “Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 350, 938–943 (2015).
Liang, Y. et al. Universal quinone electrodes for long cycle life aqueous rechargeable batteries. Nat. Mater. 16, 841–848 (2017).
Kim, H. et al. Aqueous rechargeable Li and Na ion batteries. Chem. Rev. 114, 11788–11827 (2014).
Lei, Y. et al. Unveiling the influence of electrode/electrolyte interface on the capacity fading for typical graphite-based potassium-ion batteries. Energy Storage Mater. 24, 319–328 (2020).
Su, D., McDonagh, A., Qiao, S. & Wang, G. High-capacity aqueous potassium-ion batteries for large-scale energy storage. Adv. Mater. 29, 1604007 (2017).
Zhu, K., Li, Z., Jin, T. & Jiao, L. Low defects potassium cobalt hexacyanoferrate as a superior cathode for aqueous potassium ion batteries. J. Mater. Chem. A 8, 21103–21109 (2020).
Wessells, C. D., Peddada, S. V., Huggins, R. A. & Cui, Y. Nickel hexacyanoferrate nanoparticle electrodes for aqueous sodium and potassium ion batteries. Nano Lett. 11, 5421–5425 (2011).
Jiang, L. et al. Building aqueous K-ion batteries for energy storage. Nat. Energy 4, 495–503 (2019).
Zhang, W., Pang, W. K., Sencadas, V. & Guo, Z. Understanding high-energy-density Sn4P3 anodes for potassium-ion batteries. Joule 2, 1534–1547 (2018).
Eftekhari, A., Jian, Z. & Ji, X. Potassium secondary batteries. ACS Appl. Mater. Interfaces 9, 4404–4419 (2017).
Zhou, A. et al. Hexacyanoferrate-type Prussian blue analogs: principles and advances toward high-performance sodium and potassium ion batteries. Adv. Energy Mater. 11, 2000943 (2020).
Zhang, Q. et al. Cathode materials for potassium-ion batteries: current status and perspective. Electrochem. Energy Rev. 1, 625–658 (2018).
Bie, X. et al. A novel K-ion battery: hexacyanoferrate(II)/graphite cell. J. Mater. Chem. A 5, 4325–4330 (2017).
Xue, L. et al. Low-cost high-energy potassium cathode. J. Am. Chem. Soc. 139, 2164–2167 (2017).
Zhan, C., Qiu, X., Lu, J. & Amine, K. Tuning the Mn deposition on the anode to improve the cycle performance of the Mn-based lithium ion battery. Adv. Mater. Interfaces 3, 1500856 (2016).
Liu, T. et al. Correlation between manganese dissolution and dynamic phase stability in spinel-based lithium-ion battery. Nat. Commun. 10, 4721 (2019).
Zhan, C., Wu, T., Lu, J. & Amine, K. Dissolution, migration, and deposition of transition metal ions in Li-ion batteries exemplified by Mn-based cathodes—a critical review. Energy Environ. Sci. 11, 243–257 (2018).
Xia, H. et al. A monoclinic polymorph of sodium birnessite for ultrafast and ultrastable sodium ion storage. Nat. Commun. 9, 5100 (2018).
Zuo, C. et al. Double the capacity of manganese spinel for lithium-ion storage by suppression of cooperative Jahn–Teller distortion. Adv. Energy Mater. 10, 2000363 (2020).
He, G. & Nazar, L. F. Crystallite size control of Prussian white analogues for nonaqueous potassium-ion batteries. ACS Energy Lett. 2, 1122–1127 (2017).
Ren, W. H., Chen, X. J. & Zhao, C. Ultrafast aqueous potassium-ion batteries cathode for stable intermittent grid-scale energy storage. Adv. Energy Mater. 8, 1801413 (2018).
Ling, T. et al. Atomic-level structure engineering of metal oxides for high-rate oxygen intercalation pseudocapacitance. Sci. Adv. 4, eaau6261 (2018).
Zhu, X. et al. LiMnO2 cathode stabilized by interfacial orbital ordering for sustainable lithium-ion batteries. Nat. Sustain 4, 392–401 (2020).
Schkeryantz, L. A. et al. Designing potassium battery salts through a solvent-in-anion concept for concentrated electrolytes and mimicking solvation structures. Chem. Mater. 32, 10423–10434 (2020).
Shang, Y. et al. Unconventional Mn vacancies in Mn–Fe Prussian blue analogs: suppressing Jahn–Teller distortion for ultrastable sodium storage. Chem 6, 1804–1818 (2020).
Xia, M. et al. Commercially available Prussian blue get energetic in aqueous K-ion batteries. Chem. Eng. J. 394, 124923 (2020).
Wang, M., Wang, H., Zhang, H. & Li, X. Aqueous K-ion battery incorporating environment-friendly organic compound and Berlin green. J. Energy Chem. 48, 14–20 (2020).
Lu, K. et al. High rate and stable symmetric potassium ion batteries fabricated with flexible electrodes and solid-state electrolytes. Nanoscale 10, 20754–20760 (2018).
Song, J. et al. Removal of interstitial H2O in hexacyanometallates for a superior cathode of a sodium-ion battery. J. Am. Chem. Soc. 137, 2658–2664 (2015).
Li, W. et al. Stress distortion restraint to boost the sodium ion storage performance of a novel binary hexacyanoferrate. Adv. Energy Mater. 10, 1903006 (2019).
Liu, S. et al. Manipulating the solvation structure of nonflammable electrolyte and interface to enable unprecedented stability of graphite anodes beyond 2 years for safe potassium-ion batteries. Adv. Mater. 33, 2006313 (2021).
Nakamoto, K. et al. Over 2 V aqueous sodium‐ion battery with Prussian blue‐type electrodes. Small Methods 3, 1800220 (2018).
Hou, Z. et al. An aqueous rechargeable sodium ion battery based on a NaMnO2//NaTi2(PO4)3 hybrid system for stationary energy storage. J. Mater. Chem. A 3, 1400–1404 (2015).
Wu, X. et al. Vacancy-free Prussian blue nanocrystals with high capacity and superior cyclability for aqueous sodium-ion batteries. ChemNanoMat 1, 188–193 (2015).
Gao, H. & Goodenough, J. B. An aqueous symmetric sodium-ion battery with NASICON-structured Na3MnTi(PO4)3. Angew. Chem. Int. Ed. Engl. 55, 12768–12772 (2016).
Suo, L. et al. “Water-in-salt” electrolyte makes aqueous sodium-ion battery safe green and long-lasting. Adv. Energy Mater. 7, 1701189 (2017).
Pasta, M. et al. Full open-framework batteries for stationary energy storage. Nat. Commun. 5, 3007 (2014).
Fernández-Ropero, A. J. et al. Electrochemical characterization of NaFePO4 as positive electrode in aqueous sodium-ion batteries. J. Power Sources 291, 40–45 (2015).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3968 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Acknowledgements
B.L. acknowledges support from the National Natural Science Foundation of China (grant no. U20A2047 and 51922038) and A.M.R. acknowledges funding through the NASA-EPSCoR Award no. NNH17ZHA002C.
Author information
Authors and Affiliations
Contributions
B.L. conceived the project. J.G. and B.L. developed the concept. J.G. conducted the experiments and data analysis. J.G., B.L. and A.M.R wrote the manuscript. All authors discussed and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Sustainability thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary discussion, Figs. 1–34 and Tables 1–10.
Supplementary Video 1
The STEM-EELS surface line of the initial electrode.
Supplementary Video 2
The STEM-EELS surface line of the modified electrode.
Rights and permissions
About this article
Cite this article
Ge, J., Fan, L., Rao, A.M. et al. Surface-substituted Prussian blue analogue cathode for sustainable potassium-ion batteries. Nat Sustain 5, 225–234 (2022). https://doi.org/10.1038/s41893-021-00810-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-021-00810-7
This article is cited by
-
Alkaline-based aqueous sodium-ion batteries for large-scale energy storage
Nature Communications (2024)
-
Safe electrolyte for long-cycling alkali-ion batteries
Nature Sustainability (2024)
-
Practical assessment of the energy density of potassium-ion batteries
Science China Chemistry (2024)
-
Effect of surfactants on the electrochemical performance of iron hexacyanoferrate prepared by co-precipitation route for aqueous potassium-ion storage
Journal of Nanoparticle Research (2024)
-
Progress on Transition Metal Ions Dissolution Suppression Strategies in Prussian Blue Analogs for Aqueous Sodium-/Potassium-Ion Batteries
Nano-Micro Letters (2024)