Abstract
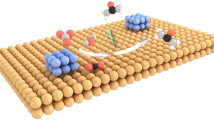
Synthetic nitrogen fertilizer such as urea has been key to increasing crop productivity and feeding a growing population. However, the conventional urea production relies on energy-intensive processes, consuming approximately 2% of annual global energy. Here, we report on a more-sustainable electrocatalytic approach that allows for direct and selective synthesis of urea from nitrate and carbon dioxide with an indium hydroxide catalyst at ambient conditions. Remarkably, Faradaic efficiency, nitrogen selectivity and carbon selectivity reach 53.4%, 82.9% and ~100%, respectively. The engineered surface semiconducting behaviour of the catalyst is found to suppress hydrogen evolution reaction. The key step of C–N coupling initiates through the reaction between *NO2 and *CO2 intermediates owing to the low energy barrier on {100} facets. This work suggests an appealing route of urea production and provides deep insight into the underlying chemistry of C–N coupling reaction that could guide sustainable synthesis of other indispensable chemicals.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data that support the findings in this paper are available within the article and its Supplementary Information. Source data are available from the corresponding author upon reasonable request.
References
Zhang, X. et al. Managing nitrogen for sustainable development. Nature 528, 51–59 (2015).
Giddey, S., Badwal, S. P. S. & Kulkarni, A. Review of electrochemical ammonia production technologies and materials. Int. J. Hydrogen Energy 38, 14576–14594 (2013).
Martín, A. J., Shinagawa, T. & Pérez-Ramírez, J. Electrocatalytic reduction of nitrogen: from Haber–Bosch to ammonia artificial leaf. Chem 5, 263–283 (2019).
Chen, J. G. et al. Beyond fossil fuel-driven nitrogen transformations. Science 360, eaar6611 (2018).
Suryanto, B. H. et al. Challenges and prospects in the catalysis of electroreduction of nitrogen to ammonia. Nat. Catal. 2, 290–296 (2019).
Hao, Y. C. et al. Promoting nitrogen electroreduction to ammonia with bismuth nanocrystals and potassium cations in water. Nat. Catal. 2, 448–456 (2019).
Lv, C. et al. Boosting electrocatalytic ammonia production through mimicking “π back-donation”. Chem 6, 2690–2702 (2020).
Wang, Y. et al. Generating defect-rich bismuth for enhancing the rate of nitrogen electroreduction to ammonia. Angew. Chem. Int. Ed. 58, 9464–9469 (2019).
Xue, Z.-H. et al. Electrochemical reduction of N2 into NH3 by donor-acceptor couples of Ni and Au nanoparticles with a 67.8% Faradaic efficiency. J. Am. Chem. Soc. 141, 14976–14980 (2019).
Fang, Z., Wu, P., Qian, Y. & Yu, G. Gel-derived amorphous bismuth–nickel alloy promotes electrocatalytic nitrogen fixation via optimizing nitrogen adsorption and activation. Angew. Chem. Int. Ed. 60, 4275–4281 (2021).
Liu, S. et al. Facilitating nitrogen accessibility to boron-rich covalent organic frameworks via electrochemical excitation for efficient nitrogen fixation. Nat. Commun. 10, 3898 (2019).
Liu, Q. et al. Crystalline red phosphorus nanoribbons: large-scale synthesis and electrochemical nitrogen fixation. Angew. Chem. Int. Ed. 59, 14383–14387 (2020).
Lv, C. et al. Defect engineering metal-free polymeric carbon nitride electrocatalyst for effective nitrogen fixation under ambient conditions. Angew. Chem. Int. Ed. 57, 10246–10250 (2018).
Chen, C. et al. Coupling N2 and CO2 in H2O to synthesize urea under ambient conditions. Nat. Chem. 12, 717–724 (2020).
Kayan, D. B. & Köleli, F. Simultaneous electrocatalytic reduction of dinitrogen and carbon dioxide on conducting polymer electrodes. Appl. Catal. B 181, 88–93 (2016).
Wu, Y., Zhang, J., Lin, Z., Liang, Y. & Wang, H. Direct electrosynthesis of methylamine from carbon dioxide and nitrate. Nat. Sustain. https://doi.org/10.1038/s41893-021-00705-7 (2021).
Chen, G.-F. et al. Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper–molecular solid catalyst. Nat. Energy 5, 605–613 (2020).
Wang, Y. et al. Enhanced nitrate-to-ammonia activity on copper–nickel alloys via tuning of intermediate adsorption. J. Am. Chem. Soc. 142, 5702–5708 (2020).
Li, J. et al. Efficient ammonia electrosynthesis from nitrate on strained ruthenium nanoclusters. J. Am. Chem. Soc. 142, 7036–7046 (2020).
Wang, Y., Zhou, W., Jia, R., Yu, Y. & Zhang, B. Unveiling the activity origin of a copper-based electrocatalyst for selective nitrate reduction to ammonia. Angew. Chem. Int. Ed. 59, 5350–5354 (2020).
Saravanakumar, D., Song, J., Lee, S., Hur, N. H. & Shin, W. Electrocatalytic conversion of carbon dioxide and nitrate ions to urea by a titania–Nafion composite electrode. ChemSusChem 10, 3999–4003 (2017).
Shibata, M. & Furuya, N. Electrochemical synthesis of urea at gas-diffusion electrodes: part VI. Simultaneous reduction of carbon dioxide and nitrite ions with various metallophthalocyanine catalysts. J. Electroanal. Chem. 507, 177–184 (2001).
Shibata, M., Yoshida, K. & Furuya, N. Electrochemical synthesis of urea on reduction of carbon dioxide with nitrate and nitrite ions using Cu-loaded gas-diffusion electrode. J. Electroanal. Chem. 387, 143–145 (1995).
Siva, P., Prabu, P., Selvam, M., Karthik, S. & Rajendran, V. Electrocatalytic conversion of carbon dioxide to urea on nano-FeTiO3 surface. Ionics 23, 1871–1878 (2017).
Feng, Y. et al. Te-doped Pd nanocrystal for electrochemical urea production by efficiently coupling carbon dioxide reduction with nitrite reduction. Nano Lett. 11, 8282–8289 (2020).
Cao, N. et al. Oxygen vacancies enhanced cooperative electrocatalytic reduction of carbon dioxide and nitrite ions to urea. J. Colloid Interface Sci. 577, 109–114 (2020).
Liu, J. et al. Controllable and facile synthesis of nearly monodisperse 18-facet indium hydroxide polyhedra. New J. Chem. 39, 1930–1937 (2015).
Yan, T. et al. Bismuth atom tailoring of indium oxide surface frustrated Lewis pairs boosts heterogeneous CO2 photocatalytic hydrogenation. Nat. Commun. 11, 6095 (2020).
Golovanov, V. et al. Experimental and theoretical studies of indium oxide gas sensors fabricated by spray pyrolysis. Sens. Actuators B 106, 563–571 (2005).
He, L. et al. Spatial separation of charge carriers in In2O3−x(OH)y nanocrystal superstructures for enhanced gas-phase photocatalytic activity. ACS Nano 10, 5578–5586 (2016).
Zhao, H., Yin, W., Zhao, M., Song, Y. & Yang, H. Hydrothermal fabrication and enhanced photocatalytic activity of hexagram shaped InOOH nanostructures with exposed {020} facets. Appl. Catal. B 130–131, 178–186 (2013).
Gurlo, A. et al. Pressure-induced decomposition of indium hydroxide. J. Am. Chem. Soc. 132, 12674–12678 (2010).
Tang, Q. et al. Size-controllable growth of single crystal In(OH)3 and In2O3 nanocubes. Cryst. Growth Des. 5, 147–150 (2005).
Bondue, C. J., Graf, M., Goyal, A. & Koper, M. T. M. Suppression of hydrogen evolution in acidic electrolytes by electrochemical CO2 reduction. J. Am. Chem. Soc. 143, 279–285 (2021).
He, Y. et al. Self-gating in semiconductor electrocatalysis. Nat. Mater. 18, 1098–1104 (2019).
Lv, C. et al. An amorphous noble-metal-free electrocatalyst that enables nitrogen fixation under ambient conditions. Angew. Chem. Int. Ed. 57, 6073–6076 (2018).
Xu, L. et al. NOx sensitivity of conductometric In(OH)3 sensors operated at room temperature and transition from p- to n-type conduction. Sens. Actuators B 245, 533–540 (2017).
Niu, T. et al. D-A-p-A-D-type dopant-free hole transport material for low-cost, efficient, and stable perovskite solar cells. Joule 5, 249–269 (2021).
Takata, T. et al. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 581, 411–414 (2020).
Yu, J., Low, J., Xiao, W., Zhou, P. & Jaroniec, M. Enhanced photocatalytic CO2-reduction activity of anatase TiO2 by coexposed {001} and {101} facets. J. Am. Chem. Soc. 136, 8839–8842 (2014).
Yue, D., Jia, Y., Yao, J., Sun, J. & Jing, Y. Structure and electrochemical behavior of ionic liquid analogue based on choline chloride and urea. Electrochim. Acta 65, 30–36 (2012).
Keuleers, R., Desseyn, H. O., Rousseau, B. & Van Alsenoy, C. Vibrational analysis of urea. J. Phys. Chem. A 103, 4621–4630 (1999).
Yao, Y., Zhu, S., Wang, H., Li, H. & Shao, M. A spectroscopic study on the nitrogen electrochemical reduction reaction on gold and platinum surfaces. J. Am. Chem. Soc. 140, 1496–1501 (2018).
Manivannan, M. & Rajendran, S. Investigation of inhibitive action of urea-Zn2+ system in the corrosion control of carbon steel in sea water. Int. J. Eng. Sci. Technol. 3, 8048–8060 (2011).
Nakamoto, K. in Handbook of Vibrational Spectroscopy Vol. 3 (eds Chalmers, J. M. & Griffiths, P.) 1872–1892 (Wiley, 2006).
Moysiadou, A., Lee, S., Hsu, C. S., Chen, H. M. & Hu, X. Mechanism of oxygen evolution catalyzed by cobalt oxyhydroxide: cobalt superoxide species as a key intermediate and dioxygen release as a rate-determining step. J. Am. Chem. Soc. 142, 11901–11914 (2020).
Rahmatullah, M. & Boyde, T. R. C. Improvements in the determination of urea using diacetyl monoxime; methods with and without deproteinisation. Clin. Chim. Acta 107, 3–9 (1980).
Zhao, Y. et al. Ammonia detection methods in photocatalytic and electrocatalytic experiments: how to improve the reliability of NH3 production rates? Adv. Sci. 6, 1802109 (2019).
Watt, G. W. & Chrisp, J. D. A spectrophotometric method for determination of hydrazine. Anal. Chem. 24, 2006–2008 (1952).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Acknowledgements
Q.Y. acknowledges funding support from Singapore MOE AcRF Tier 1 grant no. 2020-T1-001-031, Tier 2 grant no. 2017-T2-2-069 and Singapore A*STAR project A19D9a0096. G.Y. acknowledges funding support from the US Department of Energy (grant number: DE-SC0019019) and Welch Foundation Award F-1861. S.L. acknowledges financial support from the Academic Research Fund Tier 1 (RG8/20), Tier 1 (RG104/18) and computing resources from the National Supercomputing Centre Singapore. This work is also supported by the Users with Excellence programme of Hefei Science Center of CAS(2020HSC-UE003). We greatly thank the Facility for Analysis, Characterization, Testing and Simulation (FACTS) of Nanyang Technological University, Singapore, for using their TEM, SEM and XRD equipment. We acknowledge NTU Center of High Field NMR Spectroscopy and Imaging. We also thank the National Synchrotron Radiation Laboratory for help in characterizations.
Author information
Authors and Affiliations
Contributions
G.Y. and Q.Y. conceived and directed the project. C. Lv carried out key experiments and wrote the manuscript. L.Z. and S.L. performed theoretical calculations. H.L. and L.S. conducted operando SR-FTIR measurements. C.Y. carried out the design of catalysts. M.C., Y.K., J.L., C. Lee and D.L. conducted part of the characterizations. C. Lv, L.Z., Z.F., Q.Y., G.Y., G.C., L.S. and S.L. analysed the data. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Sustainability thanks Qi Shao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–67, Discussion, Table 1 and references.
Rights and permissions
About this article
Cite this article
Lv, C., Zhong, L., Liu, H. et al. Selective electrocatalytic synthesis of urea with nitrate and carbon dioxide. Nat Sustain 4, 868–876 (2021). https://doi.org/10.1038/s41893-021-00741-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-021-00741-3
This article is cited by
-
Defect-induced triple synergistic modulation in copper for superior electrochemical ammonia production across broad nitrate concentrations
Nature Communications (2024)
-
Pulsed co-electrolysis of carbon dioxide and nitrate for sustainable urea synthesis
Nature Sustainability (2024)
-
Potential and electric double-layer effect in electrocatalytic urea synthesis
Nature Communications (2024)
-
Sequential co-reduction of nitrate and carbon dioxide enables selective urea electrosynthesis
Nature Communications (2024)
-
Theoretical Study on the Synthesis of Urea by Electrochemical Nitrate and Carbon Dioxide over COF Series Catalysts
Catalysis Surveys from Asia (2024)