Abstract
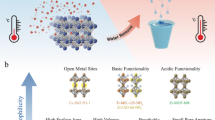
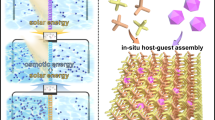
Light-responsive materials with high adsorption capacity and sunlight-triggered regenerability are highly desired for their low-cost and environmentally friendly industrial separation processes. Here we report a poly(spiropyran acrylate) (PSP) functionalized metal–organic framework (MOF) as a sunlight-regenerable ion adsorbent for sustainable water desalination. Under dark conditions, the zwitterionic isomer quickly adsorbs multiple cations and anions from water within 30 minutes, with high ion adsorption loadings of up to 2.88 mmol g−1 of NaCl. With sunlight illumination, the neutral isomer rapidly releases these adsorbed salts within 4 minutes. Single-column desalination experiments demonstrated that PSP–MOF works efficiently for water desalination. A freshwater yield of 139.5 l kg−1 d−1 and a low energy consumption of 0.11 Wh l−1 would be reached for desalinating 2,233 ppm synthetic brackish water. Importantly, this adsorbent shows excellent stability and cycling performance. This work opens up a new direction for designing stimuli-responsive materials for energy-efficient and sustainable desalination and water purification.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data supporting the findings of this study are available in the paper and its Supplementary Information files.
Change history
09 February 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41893-024-01303-z
References
Stokes, J. & Horvath, A. Life cycle energy assessment of alternative water supply systems. Int J. Life Cycle Assess. 11, 335–343 (2006).
Elimelech, M. & Phillip, W. A. The future of seawater desalination: energy, technology, and the environment. Science 333, 712–717 (2011).
Henderson‐Sellers, B. A new formula for latent heat of vaporization of water as a function of temperature. Q. J. R. Meteorol. Soc. 110, 1186–1190 (1984).
Al-Karaghouli, A. & Kazmerski, L. L. Energy consumption and water production cost of conventional and renewable-energy-powered desalination processes. Renew. Sustain. Energy Rev. 24, 343–356 (2013).
Burn, S. et al. Desalination techniques—a review of the opportunities for desalination in agriculture. Desalination 364, 2–16 (2015).
Ang, W. S., Yip, N. Y., Tiraferri, A. & Elimelech, M. Chemical cleaning of RO membranes fouled by wastewater effluent: achieving higher efficiency with dual-step cleaning. J. Memb. Sci. 382, 100–106 (2011).
Amy, G. et al. Membrane-based seawater desalination: present and future prospects. Desalination 401, 16–21 (2017).
Wang, Z. et al. Nanoarchitectured metal–organic framework/polypyrrole hybrids for brackish water desalination using capacitive deionization. Mater. Horiz. 6, 1433–1437 (2019).
Porada, S., Zhao, R., van der Wal, A., Presser, V. & Biesheuvel, P. M. Review on the science and technology of water desalination by capacitive deionization. Prog. Mater. Sci. 58, 1388–1442 (2013).
Chandrasekara, N. G. N. & Pashley, R. Study of a new process for the efficient regeneration of ion exchange resins. Desalination 357, 131–139 (2015).
Bolto, B. et al. An ion exchange process with thermal regeneration IX. A new type of rapidly reacting ion-exchange resin. Desalination 13, 269–285 (1973).
Ou, R. et al. Thermoresponsive amphoteric metal–organic frameworks for efficient and reversible adsorption of multiple salts from water. Adv. Mater. 30, 1802767 (2018).
Blankenship, R. E. et al. Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science 332, 805–809 (2011).
Ni, G. et al. Steam generation under one sun enabled by a floating structure with thermal concentration. Nat. Energy 1, 16126 (2016).
Tao, P. et al. Solar-driven interfacial evaporation. Nat. Energy 3, 1031–1041 (2018).
Chiavazzo, E., Morciano, M., Viglino, F., Fasano, M. & Asinari, P. Passive solar high-yield seawater desalination by modular and low-cost distillation. Nat. Sustain. 1, 763–772 (2018).
Scholes, G. D., Fleming, G. R., Olaya-Castro, A. & Van Grondelle, R. Lessons from nature about solar light harvesting. Nat. Chem. 3, 763–774 (2011).
Barber, J. Photosynthetic energy conversion: natural and artificial. Chem. Soc. Rev. 38, 185–196 (2009).
Li, X.-P. et al. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403, 391–395 (2000).
Ferreira, K. N., Iverson, T. M., Maghlaoui, K., Barber, J. & Iwata, S. Architecture of the photosynthetic oxygen-evolving center. Science 303, 1831–1838 (2004).
Nagel, G. et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl Acad. Sci. USA 100, 13940–13945 (2003).
Govorunova, E. G., Sineshchekov, O. A., Janz, R., Liu, X. & Spudich, J. L. Natural light-gated anion channels: a family of microbial rhodopsins for advanced optogenetics. Science 349, 647–650 (2015).
Roy, D., Cambre, J. N. & Sumerlin, B. S. Future perspectives and recent advances in stimuli-responsive materials. Prog. Polym. Sci. 35, 278–301 (2010).
Stuart, M. A. C. et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 9, 101–113 (2010).
Klajn, R. Spiropyran-based dynamic materials. Chem. Soc. Rev. 43, 148–184 (2014).
Radu, A. et al. Spiropyran-based reversible, light-modulated sensing with reduced photofatigue. J. Photochem. Photobiol. A 206, 109–115 (2009).
Furukawa, H., Cordova, K. E., O’Keeffe, M. & Yaghi, O. M. The chemistry and applications of metal–organic frameworks. Science 341, 1230444 (2013).
Bachman, J. E., Smith, Z. P., Li, T., Xu, T. & Long, J. R. Enhanced ethylene separation and plasticization resistance in polymer membranes incorporating metal–organic framework nanocrystals. Nat. Mater. 15, 845–849 (2016).
Loiseau, T. et al. A rationale for the large breathing of the porous aluminum terephthalate (MIL‐53) upon hydration. Chem. Eur. J. 10, 1373–1382 (2004).
Wang, C., Liu, X., Demir, N. K., Chen, J. P. & Li, K. Applications of water stable metal–organic frameworks. Chem. Soc. Rev. 45, 5107–5134 (2016).
Llewellyn, P. L. et al. Prediction of the conditions for breathing of metal organic framework materials using a combination of X-ray powder diffraction, microcalorimetry, and molecular simulation. J. Am. Chem. Soc. 130, 12808–12814 (2008).
Boutin, A. et al. Breathing transitions in MIL‐53 (Al) metal–organic framework upon xenon adsorption. Angew. Chem. Int. Ed. 48, 8314–8317 (2009).
Song, X., Zhou, J., Li, Y. & Tang, Y. Correlations between solvatochromism, Lewis acid–base equilibrium and photochromism of an indoline spiropyran. J. Photochem. Photobiol. A 92, 99–103 (1995).
Rosario, R., Gust, D., Hayes, M., Springer, J. & Garcia, A. A. Solvatochromic study of the microenvironment of surface-bound spiropyrans. Langmuir 19, 8801–8806 (2003).
Fissi, A., Pieroni, O., Angelini, N. & Lenci, F. Photoresponsive polypeptides: photochromic and conformational behavior of spiropyran-containing poly (L-glutamate) s under acid conditions. Macromolecules 32, 7116–7121 (1999).
Kho, Y. M. & Shin, E. J. Spiropyran-isoquinoline dyad as a dual chemosensor for Co(II) and In(III) detection. Molecules 22, 1569 (2017).
Kunin, R. Further studies on the weak electrolyte ion exchange resin desalination process (desal process). Desalination 4, 38–44 (1968).
Kunin, R. & McGarvey, F. X. Monobed deionization with ion exchange resins. Ind. Eng. Chem. 43, 734–740 (1951).
Bolto, B., Eppinger, K., Jackson, M. & Siudak, R. An ion-exchange process with thermal regeneration XIV thermally regenerable resin systems with high capacities. Desalination 34, 171–188 (1980).
Karabelas, A. J., Koutsou, C. P., Kostoglou, M. & Sioutopoulos, D. C. Analysis of specific energy consumption in reverse osmosis desalination processes. Desalination 431, 15–21 (2018).
Zhang, Y. Z. et al. Fit-for-purpose block polymer membranes molecularly engineered for water treatment. NPJ Clean Water 1, 2 (2018).
Zodrow, K. R. et al. Advanced materials, technologies, and complex systems analyses: emerging opportunities to enhance urban water security. Environ. Sci. Technol. 51, 10274–10281 (2017).
Acknowledgements
This work is supported by the Australia Research Council (project no. LP160101228). We thank the staff of the Monash Centre for Electron Microscopy (MCEM) for their technical support and assistance with the electron microscopy.
Author information
Authors and Affiliations
Contributions
R.O., H.Z. and H.W. designed the experiments. R.O. and V.X.T. performed the synthesis of the samples. R.O., V.X.T., H.M.H., J.H. and L.H. performed the characterizations. R.O. and L.Z. calculated the energy consumption. R.O., H.Z., V.X.T. and H.W. wrote the paper. G.P.S., A.D., L.Z., X.Z. and L.J. contributed to the project discussions and manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–20, Tables 1–7 and Notes 1–7.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ou, R., Zhang, H., Truong, V.X. et al. A sunlight-responsive metal–organic framework system for sustainable water desalination. Nat Sustain 3, 1052–1058 (2020). https://doi.org/10.1038/s41893-020-0590-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-020-0590-x
This article is cited by
-
Photocatalytic aerobic oxidation of C(sp3)-H bonds
Nature Communications (2024)
-
Thermodiffusive desalination
Nature Communications (2024)
-
Bioinspired light-driven chloride pump with helical porphyrin channels
Nature Communications (2024)
-
Photoelectric responsive ionic channel for sustainable energy harvesting
Nature Communications (2023)
-
Enhanced and synergistic catalytic activation by photoexcitation driven S−scheme heterojunction hydrogel interface electric field
Nature Communications (2023)