Abstract
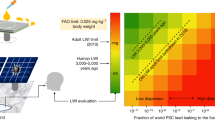
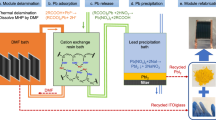
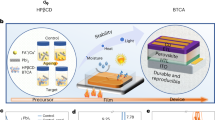
Despite the rapid development of perovskite solar cells (PSCs) toward commercialization, the toxic lead (Pb) ions in PSCs pose a potential threat to the environment, health and safety. Managing Pb via recycling represents a promising approach to mitigating its toxicity. However, managing Pb from commonly used organic solvents has been challenging due to the lack of suitable Pb adsorbents. Here, we report a new adsorbent for both separation and recovery of Pb from PSC pollutants. The synthesized iron-incorporated hydroxyapatite possesses a strongly negatively charged surface that improves electrostatic interaction through surface-charge delocalization, thus leading to enhanced Pb adsorption. We demonstrate the feasibility of a complete Pb management process, including the purification of Pb-containing non-aqueous solvents below 15 parts per 109, a level compliant with the standards of the US Environmental Protection Agency, as well as recycling of 99.97% of Pb ions by forming lead iodide.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the Article and its Supplementary Information file and from the corresponding author upon reasonable request. Any available information on data resources used in or produced for the paper is provided.
References
Jeon, N. J. et al. A fluorene-terminated hole-transporting material for highly efficient and stable perovskite solar cells. Nat. Energy 3, 682–689 (2018).
Yang, W. S. et al. Iodide management in formamidinium-lead-halide-based perovskite layers for efficient solar cells. Science 356, 1376–1379 (2017).
Christians, J. A. et al. Tailored interfaces of unencapsulated perovskite solar cells for >1,000 hour operational stability. Nat. Energy 3, 68–74 (2018).
Deng, Y. H. et al. Surfactant-controlled ink drying enables high-speed deposition of perovskite films for efficient photovoltaic modules. Nat. Energy 3, 560–566 (2018).
Yang, M. J. et al. Highly efficient perovskite solar modules by scalable fabrication and interconnection optimization. ACS Energy Lett. 3, 322–328 (2018).
Kim, D. H., Whitaker, J. B., Li, Z., van Hest, M. F. A. M. & Zhu, K. Outlook and challenges of perovskite solar cells toward terawatt-scale photovoltaic module technology. Joule 2, 1437–1451 (2018).
Babayigit, A., Ethirajan, A., Muller, M. & Conings, B. Toxicity of organometal halide perovskite solar cells. Nat. Mater. 15, 247–251 (2016).
Kim, B. J. et al. Selective dissolution of halide perovskites as a step towards recycling solar cells. Nat. Commun. 7, 11735 (2016).
Park, N.-G., Grätzel, M., Miyasaka, T., Zhu, K. & Emery, K. Towards stable and commercially available perovskite solar cells. Nat. Energy 1, 16152 (2016).
Abate, A. Perovskite solar cells go lead free. Joule 1, 659–664 (2017).
Lin-Fu, J. S. Vulnerability of children to lead exposure and toxicity (second of two parts). N. Engl. J. Med. 289, 1289–1293 (1973).
Matlock, M. M., Howerton, B. S. & Atwood, D. A. Chemical precipitation of lead from lead battery recycling plant wastewater. Ind. Eng. Chem. Res. 41, 1579–1582 (2002).
Bolisetty, S. & Mezzenga, R. Amyloid-carbon hybrid membranes for universal water purification. Nat. Nanotechnol. 11, 365–371 (2016).
Dabrowski, A., Hubicki, Z., Podkościelny, P. & Robens, E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 56, 91–106 (2004).
Ali, I. & Gupta, V. K. Advances in water treatment by adsorption technology. Nat. Protoc. 1, 2661–2667 (2006).
Zhao, J. et al. Facile preparation of a self-assembled Artemia cyst shell–TiO2–MoS2 porous composite structure with highly efficient catalytic reduction of nitro compounds for wastewater treatment. Nanotechnology 31, 085603 (2020).
Yuan, Q. L. et al. Facet-dependent selective adsorption of Mn-doped alpha-Fe2O3 nanocrystals toward heavy-metal ions. Chem. Mater. 29, 10198–10205 (2017).
Huang, X. et al. Facile preparation of hierarchical AgNP-loaded MXene/Fe3O4/polymer nanocomposites by electrospinning with enhanced catalytic performance for wastewater treatment. ACS Omega 4, 1897–1906 (2019).
Wang, C. et al. Facile preparation and catalytic performance characterization of AuNPs-loaded hierarchical electrospun composite fibers by solvent vapor annealing treatment. Colloids Surf. A 61, 283–291 (2019).
Bailliez, S., Nzihou, A., Bèche, E. & Flamant, G. Removal of lead (Pb) by hydroxyapatite sorbent. Process Saf. Environ. Prot. 82, 175–180 (2004).
Meski, S., Ziani, S. & Khireddine, H. Removal of lead ions by hydroxyapatite prepared from the egg shell. J. Chem. Eng. Data 55, 3923–3928 (2010).
Elliott, J. C. Structure and Chemistry of the Apatites and Other Calcium Orthophosphates (Elsevier, 1994).
Ignjatović, N. L. et al. Rare-earth (Gd3+,Yb3+/Tm3+, Eu3+) co-doped hydroxyapatite as magnetic, up-conversion and down-conversion materials for multimodal imaging. Sci. Rep. 9, 16305 (2019).
Lai, W. et al. Hydrothermal fabrication of porous hollow hydroxyapatite microspheres for a drug delivery system. Mat. Sci. Eng. C. 62, 166–172 (2016).
Wang, Y. S., Moo, Y. X., Chen, C., Gunawan, P. & Xu, R. Fast precipitation of uniform CaCO3 nanospheres and their transformation to hollow hydroxyapatite nanospheres. J. Colloid Interface Sci. 352, 393–400 (2010).
Park, S. Y. et al. Osteoinductive superparamagnetic Fe nanocrystal/calcium phosphate heterostructured microspheres. Nanoscale 9, 19145–19153 (2017).
Singh., N. et al. Polydopamine modified superparamagnetic iron oxide nanoparticles as multifunctional nanocarrier for targeted prostate cancer treatment. Nanomaterials 9, 138 (2019).
Iannotti, V. et al. Fe-doping-induced magnetism in nano-hydroxyapatites. Inorg. Chem. 56, 4446–44583 (2017).
Kawabata, S. et al. Synthesis and Characterization of Wet Chemically Derived Magnetite‐HAP Hybrid Nanoparticles (American Ceramic Society, 2010).
Hailegnaw, B., Kirmayer, S., Edri, E., Hodes, G. & Cahen, D. Rain on methylammonium lead iodide based perovskites: possible environmental effects of perovskite solar cells. J. Phys. Chem. Lett. 6, 1543–1547 (2015).
Lead Laws and Regulations (EPA, 2015).
Babayigit, A. et al. Assessing the toxicity of Pb- and Sn-based perovskite solar cells in model organism Danio rerio. Sci. Rep. 6, 18721 (2016).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 27, 1787–1799 (2006).
Markovic, M., Flowler, B. O. & Tung, M. S. Preparation and comprehensive characterization of a calcium hydroxyapatite reference material. J. Res. Natl Inst. Stand. Technol. 109, 553–568 (2004).
Acknowledgements
This work was supported by the Global Frontier R&D Program on Center for Multiscale Energy System funded by the National Research Foundation (under contract no. 2012M3A6A7054855), the Alchemist project of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) granted financial resource from the Ministry of Trade, Industry & Energy (20193091010310), and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2018M3C1B7021994 and 2019R1F1A1064095). This research was partially supported by the Ministry of Science, ICT and the Future Planning as Global Frontier Project (CAMM-2019M3A6B3030638). This research was also supported by the Defense Challengeable Future Technology Program of the Agency for Defense Development, Republic of Korea. The work at the National Renewable Energy Laboratory (NREL) was supported by the De-Risking Halide Perovskite Solar Cells programme of the National Center for Photovoltaics, funded by the Office of Energy Efficiency and Renewable Energy, Solar Energy Technologies Office, US Department of Energy (DOE) under contract no. DE-AC36-08GO28308 with the Alliance for Sustainable Energy, a Limited Liability Company (LLC), and the Manager and Operator of NREL. The views expressed in the article do not necessarily represent the views of the DOE or the US Government. Via our membership of the UK’s HEC Materials Chemistry Consortium, which is funded by EPSRC (EP/L000202), this work used the ARCHER UK National Supercomputing Service (http://www.archer.ac.uk).
Author information
Authors and Affiliations
Contributions
H.S.J. and D.H.K. supervised this work. S.Y.P. and D.H.K. conceived the idea and designed the experiments. S.Y.P., D.H.K. and J.-S.P. discussed the mechanism and designed the experiment and theoretical calculations. S.Y.P. carried out the synthesis and characterization of materials and the Pb-management test. S.Y.P. and H.L. conducted the magnetic analysis of materials. J.-S.P. and A.W. designed and performed the theoretical calculations. B.J.K., D.H.K. and K.Z. performed the device fabrication and analysis. S.Y.P., J.-S.P., K.Z., D.H.K. and H.S.J. wrote the first draft of the manuscript, and all authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–15, Tables 1–3 and refs. 1–10.
Supplementary Video 1
Protocol of Pb-purification in non-aqueous solvent using HAP/Fe composites.
Rights and permissions
About this article
Cite this article
Park, S.Y., Park, JS., Kim, B.J. et al. Sustainable lead management in halide perovskite solar cells. Nat Sustain 3, 1044–1051 (2020). https://doi.org/10.1038/s41893-020-0586-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-020-0586-6
This article is cited by
-
Structurally Flexible 2D Spacer for Suppressing the Electron–Phonon Coupling Induced Non-Radiative Decay in Perovskite Solar Cells
Nano-Micro Letters (2024)
-
Bioinspired “cage traps” for closed-loop lead management of perovskite solar cells under real-world contamination assessment
Nature Communications (2023)
-
Lead immobilization for environmentally sustainable perovskite solar cells
Nature (2023)
-
Reducing lead toxicity of perovskite solar cells with a built-in supramolecular complex
Nature Sustainability (2023)
-
Spherical hydroxyapatite nanoparticle scaffolds for reduced lead release from damaged perovskite solar cells
Communications Materials (2022)