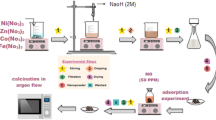
Abstract
Producing hydrogen in clean, affordable and safe manners without damaging the environment can help address the challenge of meeting a growing energy demand sustainably. Yeast biomass-derived materials—such as multi-heteroatoms (nitrogen, sulfur and phosphorus) doped carbon (MHC) catalysts from waste biomass—can help develop efficient, eco-friendly and economical catalysts to improve the sustainability of hydrogen production. Here we report hydrogen and oxygen production in 1 M potassium hydroxide using ruthenium single atoms (RuSAs) along with Ru nanoparticles (RuNPs) embedded in MHC (RuSAs + RuNPs@MHC) as a cathode and magnetite (Fe3O4) supported on MHC (Fe3O4@MHC) as an anode. The RuSAs + RuNPs@MHC catalyst outperforms the state-of-the-art commercial platinum on carbon catalyst for hydrogen evolution reaction in terms of overpotential, exchange current density, Tafel slope and durability. Furthermore, compared with industrially adopted catalysts (that is, iridium oxide), the Fe3O4@MHC catalyst displays outstanding oxygen evolution reaction activity. For whole water splitting, it requires a solar voltage of 1.74 V to drive ~ 30 mA, along with remarkable long-term stability in the presence (12 h) and absence (58 h) of outdoor-sunlight exposure, as a promising strategy towards a sustainable energy development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the article, its Supplementary Information file and from the corresponding author upon reasonable request. Source Data are provided with this paper.
References
Blakemore, J. D., Crabtree, R. H. & Brudvig, G. W. Molecular catalysts for water oxidation. Chem. Rev. 115, 12974–13005 (2015).
Peng, W. et al. Managing China’s coal power plants to address multiple environmental objectives. Nat. Sustain. 1, 693–701 (2018).
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017).
World Energy Outlook 2015 (International Energy Agency, 2015).
Song, F. et al. Transition metal oxides as electrocatalysts for the oxygen evolution reaction in alkaline solutions: an application-inspired renaissance. J. Am. Chem. Soc. 140, 7748–7759 (2018).
Tiwari, J. N. et al. Multicomponent electrocatalyst with ultralow Pt loading and high hydrogen evolution activity. Nat. Energy 3, 773–782 (2018).
Nørskov, J. K., Bligaard, T., Rossmeisl, J. & Christensen, C. H. Towards the computational design of solid catalysts. Nat. Chem. 1, 37–46 (2009).
Hunter, B. M., Gray, H. B. & Müller, A. M. Earth-abundant heterogeneous water oxidation catalysts. Chem. Rev. 116, 14120–14136 (2016).
Sultan, S. et al. Superb water splitting activity of the electrocatalyst Fe3Co(PO4)4 designed with computation aid. Nat. Commun. 10, 5195 (2019).
Georgakilas, V., Perman, J. A., Tucek, J. & Zboril, R. Broad family of carbon nanoallotropes: classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem. Rev. 115, 4744–4822 (2015).
Goldfarb, J. L., Dou, G., Salari, M. & Grinstaff, M. W. Biomass-based fuels and activated carbon electrode materials: an integrated approach to green energy systems. ACS Sustain. Chem. Eng. 5, 3046–3054 (2017).
Wu, X.-L. et al. Biomass-derived sponge-like carbonaceous hydrogels and aerogels for supercapacitors. ACS Nano 7, 3589–3597 (2013).
Schneegurt, M. A. et al. Biomass byproducts for the remediation of wastewaters contaminated with toxic metals. Environ. Sci. Technol. 35, 3786–3791 (2001).
Aguilar-Uscanga, B. & François, J. M. A study of the yeast cell wall composition and structure in response to growth conditions and mode of cultivation. Lett. Appl. Microbiol. 37, 268–274 (2003).
Morgan, D. J. Resolving ruthenium: XPS studies of common ruthenium materials. Surf. Interface Anal. 47, 1072–1079 (2015).
Li, R., Wei, Z. & Gou, X. Nitrogen and phosphorus dual-doped graphene/carbon nanosheets as bifunctional electrocatalysts for oxygen reduction and evolution. ACS Catal. 5, 4133–4142 (2015).
Qie, L. et al. Sulfur-doped carbon with enlarged interlayer distance as a high-performance anode material for sodium-ion batteries. Adv. Sci. 2, 1500195 (2015).
Peng, H. et al. High performance Fe- and N- doped carbon catalyst with graphene structure for oxygen reduction. Sci. Rep. 3, 1765 (2013).
Song, P., Zhang, Y., Pan, J., Zhuang, L. & Xu, W. Cheap carbon black-based high-performance electrocatalysts for oxygen reduction reaction. Chem. Commun. 51, 1972–1975 (2015).
Yang, F., Chi, C., Wang, C. X., Wang, Y. & Li, Y. F. High graphite N content in nitrogen-doped graphene as an efficient metal-free catalyst for reduction of nitroarenes in water. Green Chem. 18, 4254–4262 (2016).
Sultan, S. et al. Highly efficient oxygen reduction reaction activity of graphitic tube encapsulating nitrided CoxFey alloy. Adv. Energy Mater. 8, 1801002 (2018).
McCrory, C. C. L. et al. Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J. Am. Chem. Soc. 137, 4347–4357 (2015).
Voiry, D. et al. The role of electronic coupling between substrate and 2D MoS2 nanosheets in electrocatalytic production of hydrogen. Nat. Mater. 15, 1003–1009 (2016).
Zheng, Y. et al. Toward design of synergistically active carbon-based catalysts for electrocatalytic hydrogen evolution. ACS Nano 8, 5290–5296 (2014).
Duan, J., Chen, S., Jaroniec, M. & Qiao, S. Heteroatom-doped graphene-based materials for energy-relevant electrocatalytic processes. ACS Catal. 5, 5207–5234 (2015).
Jiao, Y., Zeng, Y., Davey, K. & Qiao, S.-Z. Activity origin and catalyst design principles for electrocatalytic hydrogen evolution on heteroatom-doped graphene. Nat. Energy 1, 16130 (2016).
Qu, K. et al. Promotion of electrocatalytic hydrogen evolution reaction on nitrogen-doped carbon nanosheets with secondary heteroatoms. ACS Nano 11, 7293–7300 (2017).
Sultan, S. et al. Single atoms and clusters based nanomaterials for hydrogen evolution, oxygen evolution reactions, and full water splitting. Adv. Energy Mater. 9, 1900624–1900671 (2019).
Wu, R. et al. NaCl protected synthesis of 3D hierarchical metal-free porous nitrogen-doped carbon catalysts for the oxygen reduction reaction in acidic electrolyte. Chem. Commun. 55, 9023–9026 (2019).
Zhang, J. et al. Heteroatom (nitrogen/sulfur)-doped graphene as an efficient electrocatalyst for oxygen reduction and evolution reactions. Catalysts 8, 475–484 (2018).
Chai, G.-L. et al. Active sites engineering leads to exceptional ORR and OER bifunctionality in P,N co-doped graphene frameworks. Energy Environ. Sci. 10, 1186–1195 (2017).
Cao, J. M., Feng, Y. Q., Liu, B. Y. & Li, H. G. Carbon skeleton doped with Co, N, S and P as efficient electrocatalyst for oxygen evolution reaction. Sci. China Mater. 61, 686–696 (2018).
Davodi, F., Tavakkoli, M., Lahtinen, J. & Kallio, T. Straightforward synthesis of nitrogen-doped carbon nanotubes as highly active bifunctional electrocatalysts for full water splitting. J. Catal. 353, 19–27 (2017).
Yang, H. B. et al. Identification of catalytic sites for oxygen reduction and oxygen evolution in N-doped graphene materials: development of highly efficient metal-free bifunctional electrocatalyst. Sci. Adv. 2, e1501122 (2016).
Kang, Z. et al. Engineering an Earth-abundant element-based bifunctional electrocatalyst for highly efficient and durable overall water splitting. Adv. Funct. Mater. 29, 1807031 (2019).
Zhang, X. et al. Highly active core–shell carbon/NiCo2O4 double microtubes for efficient oxygen evolution reaction: ultralow overpotential and superior cycling stability. Small 15, 1903297 (2019).
Zhang, W., Wu, Y., Qi, J., Chen, M. & Cao, R. A thin NiFe hydroxide film formed by stepwise electrodeposition strategy with significantly improved catalytic water oxidation efficiency. Adv. Energy Mater. 7, 1602547 (2017).
Campos-Roldán, C. A., González-Huerta, R. G. & Alonso-Vante, N. Experimental protocol for HOR and ORR in alkaline electrochemical measurements. J. Electrochem. Soc. 165, J3001–J3007 (2018).
Acknowledgements
This work was supported by NRF (National Honor Scientist Program: 2010-0020414). We appreciate the use of the beamline 6D at the Pohang Accelerator Laboratory (PAL).
Author information
Authors and Affiliations
Contributions
J.N.T. planned the experiment, physical characterization and electrochemical measurements and analysed the data. N.K.D. synthesized the catalysts and electrochemical measurements. S.S. discussed the results. P.T. performed full water splitting and data analysis. H.Y.J. further refined STEM measurements. J.N.T. and K.S.K. wrote the manuscript. K.S.K. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Photographs for large-scale synthesis of catalysts using yeast biomass.
a, R-2 (RuSAs+RuNPs@MHC)40mg. b, F-2 (Fe3O4@MHC)1.4g.
Extended Data Fig. 2 SEM images.
SEM images of (a) R-0 (without glutaraldehyde/NaCl) (b) R-4 (without glutaraldehyde), (c) R-5 (without hydrothermal process), and (d) R-2 (with glutaraldehyde/NaCl). Scale bars in 2 µm.
Extended Data Fig. 3 High-angle-annular-dark-field (HAADF) image.
High-angle-annular-dark-field (HAADF) image of R-2 and the corresponding energy-dispersive X-ray spectroscopy (EDS) elemental mapping images show that the sample contains C, Ru, S, N and P. Scale bars in 1 µm.
Extended Data Fig. 4 X-ray photoelectron spectroscopy (XPS) spectra.
a, XPS full survey spectrum of the R-2 sample and the corresponding core-level spectra of Ru 2p (b), C 1s (c), P 2p (d), S 2p (e), and N 1s (f).
Extended Data Fig. 5 X-ray diffraction (XRD) spectra of synthesized catalysts with different amount of Ru precursors.
a, R-1(RuSAs+RuNPs@MHC)35mg. b, R-2 (RuSAs+RuNPs@MHC)40mg. c, R-3 (RuNPs@MHC)45mg. For each spectrum, all diffraction peaks at 38.4°, 42.2°, 44.0°, 58.3°, 69.4°, 78.4°, and 84.7° correspond to the (100), (002), (101), (102), (110), (103), and (112) planes of a hexagonal-close packed (hcp) Ru crystal (JCPDS 03-065-7646). It confirms that the hcp Ru crystal structure remains unaffected after using the various amount of Ru precursors. However, the peak shape is strongly affected by Ru precursors. When we used a higher amount of Ru precursor (R-3), the peak broadening decreases and the sharpness of the peak increases, indicative of increase in crystallite size.
Extended Data Fig. 6 X-ray photoelectron spectroscopy (XPS) spectra.
a, XPS full survey spectrum of R-1 sample and the corresponding core-level spectra of Ru 2p (b), C 1s (c), P 2p (d), S 2p (e), and N 1s (f).
Extended Data Fig. 7 X-ray photoelectron spectroscopy (XPS) spectra.
a, XPS full survey spectrum of R-3 sample and the corresponding core-level spectra of Ru 2p (b), C 1s (c), P 2p (d), S 2p (e), and N 1s (f).
Extended Data Fig. 8 SEM and TEM images of R-1.
a, SEM image. b, TEM image of carbonized individual yeast cells. c, HRTEM image taken from the top edge of the carbonized individual yeast cells in (b). Inset shows the particle size distribution of RuNPs. d, AC-HAADF-STEM image showing the presence of single atoms and NPs in R-1. e, High-angle-annular-dark-field (HAADF) image and the corresponding energy-dispersive X-ray spectroscopy (EDS) elemental mapping images show that the sample contains C, Ru, N, P and S. Scale bars in (e): 1 µm. When we use 35 mg Ru precursor, the single atoms (SAs) are easily detected.
Extended Data Fig. 9 SEM and TEM images of R-3.
a, SEM image. b, TEM image of carbonized individual yeast cells. c, HRTEM image taken from the top edge of the carbonized individual yeast cells in (b). Inset shows the particle size distribution of RuNPs. d, AC-HAADF-STEM image showing the absence of single atoms R-3. e, High-angle-annular-dark-field (HAADF) image and the corresponding energy-dispersive X-ray spectroscopy (EDS) elemental mapping images show that the sample contains C, Ru, N, P and S. Scale bars in (e): 1 µm. When we use 45 mg Ru precursor, the single atoms (SAs) are fully disappeared due to aggration of nanoparticles.
Extended Data Fig. 10 TEM and HAADF images of Ru/GO.
a, TEM image. b, HRTEM image. c, High-angle-annular-dark-field (HAADF) image and the corresponding energy-dispersive X-ray spectroscopy (EDS) elemental mapping images show that the sample contains C and Ru. Scale bars in (c): 500 nm.
Supplementary information
Supplementary Information
Supplementary Figs. 1–17, Tables 1–3, Note 1 and methods.
Supplementary Video 1
Demonstration of full water splitting by solar panel in alkaline electrolyte.
Source data
Source Data Fig. 2
Unmodified experimental electron energy loss spectra (EELS) data.
Rights and permissions
About this article
Cite this article
Tiwari, J.N., Dang, N.K., Sultan, S. et al. Multi-heteroatom-doped carbon from waste-yeast biomass for sustained water splitting. Nat Sustain 3, 556–563 (2020). https://doi.org/10.1038/s41893-020-0509-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-020-0509-6
This article is cited by
-
Efficient osmosis-powered production of green hydrogen
Nature Sustainability (2024)
-
Revealing the role of interfacial water and key intermediates at ruthenium surfaces in the alkaline hydrogen evolution reaction
Nature Communications (2023)
-
Single-Atom Catalysts: Advances and Challenges in Metal-Support Interactions for Enhanced Electrocatalysis
Electrochemical Energy Reviews (2022)
-
Hydrangea flower-like nanostructure of dysprosium-doped Fe-MOF for highly efficient oxygen evolution reaction
Rare Metals (2022)
-
Sustained CO2-photoreduction activity and high selectivity over Mn, C-codoped ZnO core-triple shell hollow spheres
Nature Communications (2021)