Abstract
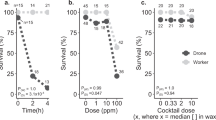
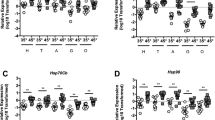
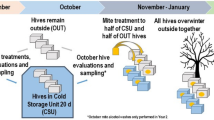
All species need to reproduce to maintain viable populations, but heat stress kills sperm cells across the animal kingdom and rising frequencies of heat waves are a threat to biodiversity. Honey bees (Apis mellifera) are globally distributed microlivestock; therefore, they could serve as environmental biomonitors for fertility losses. Here, we found that queens have two potential routes of temperature-stress exposure: within colonies and during routine shipping. Our data suggest that temperatures of 15–38 °C are safe for queens at a tolerance threshold of 11.5% loss of sperm viability, which is the viability difference associated with queen failure in the field. Heat shock activates expression of specific stress-response proteins in the spermatheca, which could serve as molecular biomarkers (indicators) for heat stress. This protein fingerprint may eventually enable surveys for the prevalence of heat-induced loss of sperm viability in diverse landscapes as part of a biomonitoring programme.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All raw mass spectrometry data, protein databases and search results are available on PRIDE ProteomeXchange (accession: PXD013728). Figures with associated raw mass spectrometry data include Figs. 4–6 and Supplementary Fig. 4. Global protein abundances and P values for the laboratory heat-shock comparisons are available in Supplementary Data 1. Source Data for Figs. 1 and 2 are provided as Source Data files. Any other data that support the findings of this study are available from the corresponding author on request.
Code availability
No specialized code central to our conclusions was used in this manuscript. R code for standard statistical analyses and figure generation will be provided upon request.
References
Bellard, C., Bertelsmeier, C., Leadley, P., Thuiller, W. & Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 15, 365–377 (2012).
Thomas, C. D. et al. Extinction risk from climate change. Nature 427, 145–148 (2004).
Bálint, M. et al. Cryptic biodiversity loss linked to global climate change. Nat. Clim. Change 1, 313–318 (2011).
Sales, K. et al. Experimental heatwaves compromise sperm function and cause transgenerational damage in a model insect. Nat. Commun. 9, 4771 (2018).
Walsh, B. S. et al. The impact of climate change on fertility. Trends Ecol. Evol. 34, 249–259 (2019).
Zeh, J. A. et al. Degrees of disruption: projected temperature increase has catastrophic consequences for reproduction in a tropical ectotherm. Glob. Change Biol. 18, 1833–1842 (2012).
Jannes, P. et al. Male subfertility induced by acute scrotal heating affects embryo quality in normal female mice. Hum. Reprod. 13, 372–375 (1998).
Pérez-Crespo, M., Pintado, B. & Gutiérrez-Adán, A. Scrotal heat stress effects on sperm viability, sperm DNA integrity, and the offspring sex ratio in mice. Mol. Reprod. Dev. 75, 40–47 (2008).
Setchell, B. The effects of heat on the testes of mammals. Anim. Reprod. 3, 81–91 (2006).
Thonneau, P., Bujan, L., Multigner, L. & Mieusset, R. Occupational heat exposure and male fertility: a review. Hum. Reprod. 13, 2122–2125 (1998).
Yaeram, J., Setchell, B. P. & Maddocks, S. Effect of heat stress on the fertility of male mice in vivo and in vitro. Reprod. Fertil. Dev. 18, 647–653 (2006).
Hurley, L. L., McDiarmid, C. S., Friesen, C. R., Griffith, S. C. & Rowe, M. Experimental heatwaves negatively impact sperm quality in the zebra finch. Proc. R. Soc. B 285, 20172547 (2018).
Breckels, R. D. & Neff, B. D. The effects of elevated temperature on the sexual traits, immunology and survivorship of a tropical ectotherm. J. Exp. Biol. 216, 2658–2664 (2013).
Harvey, S. C. & Viney, M. E. Thermal variation reveals natural variation between isolates of Caenorhabditis elegans. J. Exp. Zool. B Mol. Dev. Evol. 308, 409–416 (2007).
Porcelli, D., Gaston, K. J., Butlin, R. K. & Snook, R. R. Local adaptation of reproductive performance during thermal stress. J. Evol. Biol. 30, 422–429 (2017).
Gasparini, C., Lu, C., Dingemanse, N. J. & Tuni, C. Paternal-effects in a terrestrial ectotherm are temperature dependent but no evidence for adaptive effects. Funct. Ecol. 32, 1011–1021 (2018).
Saxena, B., Sharma, P., Thappa, R. & Tikku, K. Temperature induced sterilization for control of three stored grain beetles. J. Stored Prod. Res. 28, 67–70 (1992).
Pettis, J. S., Rice, N., Joselow, K., vanEngelsdorp, D. & Chaimanee, V. Colony failure linked to low sperm viability in honey bee (Apis mellifera) queens and an exploration of potential causative factors. PLoS ONE 11, e0147220 (2016).
Stürup, M., Baer-Imhoof, B., Nash, D. R., Boomsma, J. J. & Baer, B. When every sperm counts: factors affecting male fertility in the honeybee Apis mellifera. Behav. Ecol. 24, 1192–1198 (2013).
Zizzari, Z. V. & Ellers, J. Effects of exposure to short-term heat stress on male reproductive fitness in a soil arthropod. J. Insect Physiol. 57, 421–426 (2011).
David, J. R. et al. Male sterility at extreme temperatures: a significant but neglected phenomenon for understanding Drosophila climatic adaptations. J. Evol. Biol. 18, 838–846 (2005).
Hansen, P. J. Effects of heat stress on mammalian reproduction. Philos. Trans. R. Soc. Lond. B 364, 3341–3350 (2009).
Gong, Y. et al. Heat stress reduces sperm motility via activation of glycogen synthase kinase-3α and inhibition of mitochondrial protein import. Front. Physiol. 8, 718 (2017).
Yang, R. C., Shen, M. R., Chiang, P. H., Yang, S. L. & Chen, S. S. A possible role of heat shock proteins in human sperm motility. Gaoxiong Yi Xue Ke Xue Za Zhi 8, 299–305 (1992).
Luber, G. & McGeehin, M. Climate change and extreme heat events. Am. J. Prev. Med. 35, 429–435 (2008).
Hayhoe, K., Sheridan, S., Kalkstein, L. & Greene, S. Climate change, heat waves, and mortality projections for Chicago. J. Great Lakes Res. 36, 65–73 (2010).
Meehl, G. A. & Tebaldi, C. More intense, more frequent, and longer lasting heat waves in the 21st century. Science 305, 994–997 (2004).
Potts, S. G. et al. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353 (2010).
Hallmann, C. A. et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 12, e0185809 (2017).
Cameron, S. A. et al. Patterns of widespread decline in North American bumble bees. Proc. Natl Acad. Sci. USA 108, 662–667 (2011).
Rasmont, P. & Mersch, P. First estimation of faunistic drift by bumblebees of Belgium (Hymenoptera: Apidae). Ann. Soc. R. Zool. Belg. 118, 141–147 (1988).
Winfree, R., Aguilar, R., Vázquez, D. P., LeBuhn, G. & Aizen, M. A. A meta-analysis of bees’ responses to anthropogenic disturbance. Ecology 90, 2068–2076 (2009).
Ricketts, T. H. et al. Landscape effects on crop pollination services: are there general patterns? Ecol. Lett. 11, 499–515 (2008).
Biesmeijer, J. C. et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313, 351–354 (2006).
Whitehorn, P. R., Tinsley, M. C., Brown, M. J., Darvill, B. & Goulson, D. Genetic diversity, parasite prevalence and immunity in wild bumblebees. Proc. R. Soc. Lond. B 278, 1195–1202 (2010).
McCallum, H. & Dobson, A. Disease, habitat fragmentation and conservation. Proc. Biol. Sci. 269, 2041–2049 (2002).
Baer, B., Collins, J., Maalaps, K. & den Boer, S. P. Sperm use economy of honeybee (Apis mellifera) queens. Ecol. Evol. 6, 2877–2885 (2016).
Delaney, D. A., Keller, J. J., Caren, J. R. & Tarpy, D. R. The physical, insemination, and reproductive quality of honey bee queens (Apis mellifera L.). Apidologie 42, 1–13 (2011).
Tarpy, D. R. & Olivarez, R. Jr Measuring sperm viability over time in honey bee queens to determine patterns in stored-sperm and queen longevity. J. Apic. Res. 53, 493–495 (2014).
Stabentheiner, A., Kovac, H. & Brodschneider, R. Honeybee colony thermoregulation–regulatory mechanisms and contribution of individuals in dependence on age, location and thermal stress. PLoS ONE 5, e8967 (2010).
Alattal, Y. Impact of temperature extremes on survival of indigenous and exotic honey bee subspecies, Apis mellifera, under desert and semiarid climates. Bull. Insectol. 68, 219–222 (2015).
Fahrenholz, L., Lamprecht, I. & Schricker, B. Thermal investigations of a honey bee colony: thermoregulation of the hive during summer and winter and heat production of members of different bee castes. J. Comp. Physiol. B 159, 551–560 (1989).
Bordier, C. et al. Colony adaptive response to simulated heat waves and consequences at the individual level in honeybees (Apis mellifera). Sci. Rep. 7, 3760 (2017).
Villa, J. D., Gentry, C. & Taylor, O. R. Preliminary observations on thermoregulation, clustering, and energy utilization in African and European honey bees. J. Kans. Entomol. Soc. 60, 4–14 (1987).
Smith, K. E. et al. Honey as a biomonitor for a changing world. Nat. Sustain. 2, 223–232 (2019).
Mitchell, J. D., Hewitt, P. & Van Der Linde, T. D. K. Critical thermal limits and temperature tolerance in the harvester termite Hodotermes mossambicus (Hagen). J. Insect Physiol. 39, 523–528 (1993).
Clémencet, J., Cournault, L., Odent, A. & Doums, C. Worker thermal tolerance in the thermophilic ant Cataglyphis cursor (Hymenoptera, Formicidae). Insectes Soc. 57, 11–15 (2010).
Baudier, K. M., Mudd, A. E., Erickson, S. C. & O’Donnell, S. Microhabitat and body size effects on heat tolerance: implications for responses to climate change (army ants: Formicidae, Ecitoninae). J. Anim. Ecol. 84, 1322–1330 (2015).
Chirault, M. et al. A combined approach to heat stress effect on male fertility in Nasonia vitripennis: from the physiological consequences on spermatogenesis to the reproductive adjustment of females mated with stressed males. PLoS ONE 10, e0120656 (2015).
Hidalgo, K., Beaugeard, E., Renault, D., Dedeine, F. & Lécureuil, C. Physiological and biochemical responses to thermal stress vary among genotypes in the parasitic wasp Nasonia vitripennis. J. Insect Physiol. 117, 103909 (2019).
Macías-Macías, J. O. et al. Comparative temperature tolerance in stingless bee species from tropical highlands and lowlands of Mexico and implications for their conservation (Hymenoptera: Apidae: Meliponini). Apidologie 42, 679–689 (2011).
Oberg, E., Del Toro, I. & Pelini, S. Characterization of the thermal tolerances of forest ants of New England. Insectes Soc. 59, 167–174 (2012).
Verble-Pearson, R. M., Gifford, M. E. & Yanoviak, S. P. Variation in thermal tolerance of North American ants. J. Therm. Biol. 48, 65–68 (2015).
Andrew, N. R., Hart, R. A., Jung, M.-P., Hemmings, Z. & Terblanche, J. S. Can temperate insects take the heat? A case study of the physiological and behavioural responses in a common ant, Iridomyrmex purpureus (Formicidae), with potential climate change. J. Insect Physiol. 59, 870–880 (2013).
Abou-Shaara, H. F., Al-Ghamdi, A. A. & Mohamed, A. A. Tolerance of two honey bee races to various temperature and relative humidity gradients. Environ. Exp. Biol. 10, 133–138 (2012).
Scaccini, D., Duso, C. & Pozzebon, A. Lethal effects of high temperatures on brown marmorated stink bug adults before and after overwintering. Insects 10, 355 (2019).
Paynter, E. et al. Insights into the molecular basis of long-term storage and survival of sperm in the honeybee (Apis mellifera). Sci. Rep. 7, 40236 (2017).
Collins, A., Williams, V. & Evans, J. Sperm storage and antioxidative enzyme expression in the honey bee, Apis mellifera. Insect Mol. Biol. 13, 141–146 (2004).
Weirich, G. F., Collins, A. M. & Williams, V. P. Antioxidant enzymes in the honey bee, Apis mellifera. Apidologie 33, 3–14 (2002).
Wagner, H., Cheng, J. W. & Ko, E. Y. Role of reactive oxygen species in male infertility: an updated review of literature. Arab J. Urol. 16, 35–43 (2018).
Agarwal, A., Virk, G., Ong, C. & du Plessis, S. S. Effect of oxidative stress on male reproduction. World J. Men’s Health 32, 1–17 (2014).
Ikwegbue, P. C., Masamba, P., Oyinloye, B. E. & Kappo, A. P. Roles of heat shock proteins in apoptosis, oxidative stress, human inflammatory diseases, and cancer. Pharmaceuticals 11, 2 (2018).
Ren, X., Chen, X., Wang, Z. & Wang, D. Is transcription in sperm stationary or dynamic? J. Reprod. Dev. 63, 439–443 (2017).
Mayer, M. P. & Bukau, B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell. Mol. Life Sci. 62, 670–684 (2005).
Yue, L. et al. Genetic analysis of viable Hsp90 alleles reveals a critical role in Drosophila spermatogenesis. Genetics 151, 1065–1079 (1999).
Ji, Z.-L. et al. Association of heat shock proteins, heat shock factors and male infertility. Asian Pac. J. Reprod. 1, 76–84 (2012).
Bakthisaran, R., Tangirala, R. & Rao, C. M. Small heat shock proteins: role in cellular functions and pathology. Biochim. Biophys. Acta 1854, 291–319 (2015).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Morrow, G. & Tanguay, R. M. Drosophila melanogaster Hsp22: a mitochondrial small heat shock protein influencing the aging process. Front. Genet. 6, 1026 (2015).
Wójtowicz, I. et al. Drosophila small heat shock protein CryAB ensures structural integrity of developing muscles, and proper muscle and heart performance. Development 142, 994–1005 (2015).
Kamradt, M. C., Chen, F. & Cryns, V. L. The small heat shock protein alpha B-crystallin negatively regulates cytochrome c- and caspase-8-dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. J. Biol. Chem. 276, 16059–16063 (2001).
Paul, C. et al. Hsp27 as a negative regulator of cytochrome C release. Mol. Cell. Biol. 22, 816–834 (2002).
Izu, H. et al. Heat shock transcription factor 1 is involved in quality-control mechanisms in male germ cells. Biol. Reprod. 70, 18–24 (2004).
Rockett, J. C. et al. Effects of hyperthermia on spermatogenesis, apoptosis, gene expression, and fertility in adult male mice. Biol. Reprod. 65, 229–239 (2001).
Biggiogera, M. et al. Localization of heat shock proteins in mouse male germ cells: an immunoelectron microscopical study. Exp. Cell. Res. 229, 77–85 (1996).
Zhang, X. S. et al. Dedifferentiation of adult monkey Sertoli cells through activation of extracellularly regulated kinase 1/2 induced by heat treatment. Endocrinology 147, 1237–1245 (2006).
Collins, A. & Donoghue, A. Viability assessment of honey bee, Apis mellifera, sperm using dual fluorescent staining. Theriogenology 51, 1513–1523 (1999).
Cobey, S. W., Tarpy, D. R. & Woyke, J. Standard methods for instrumental insemination of Apis mellifera queens. J. Apic. Res. 52, 1–18 (2013).
Taylor, C. M., Coffey, P. L., Hamby, K. A. & Dively, G. P. Laboratory rearing of Halyomorpha halys: methods to optimize survival and fitness of adults during and after diapause. J. Pest Sci. 90, 1069–1077 (2017).
Mackensen, O. Effect of carbon dioxide on initial oviposition of artificially inseminated and virgin queen bees. J. Econ. Entomol. 40, 344–349 (2014).
Rappsilber, J., Ishihama, Y. & Mann, M. Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal. Chem. 75, 663–670 (2003).
McAfee, A., Chan, Q., Evans, J. & Foster, L. J. A Varroa destructor protein atlas reveals molecular underpinnings of developmental transitions and sexual differentiation. Mol. Cell. Proteom. 16, 2125–2137 (2017).
Lee, H. K., Braynen, W., Keshav, K. & Pavlidis, P. ErmineJ: tool for functional analysis of gene expression data sets. BMC Bioinf. 6, 269 (2005).
McAfee, A., Pettis, J. S., Tarpy, D. R. & Foster, L. J. Feminizer and doublesex knock-outs cause honey bees to switch sexes. PLoS Biol. 17, e3000256 (2019).
Acknowledgements
This work was supported by Natural Sciences and Engineering Research Council of Canada Discovery grant no. 311654-11 and grants from Genome Canada and Genome British Columbia awarded to L.J.F.; Project Apis m grants awarded to A.M., M.M.G. and J.S.P.; and USDA-NIFA grant no. 2016-07962 awarded to J.S.P. and D.R.T. We thank A. Sébastien and M. Jelen for providing us with stink bugs and fruit flies, respectively, for the survival experiments. We also thank Ashurst Bee Company for help with colony heat testing and Kettle Valley Queens, Nicola Valley Honey, Wild Antho, Campbells Gold Honey, Heather Meadows Honey Farm, Six Legs Good Apiaries, Wildwood Queens, Cariboo Honey and Worker Bee Honey Company for donating failed and healthy queens for this research.
Author information
Authors and Affiliations
Contributions
A.M. wrote the first draft of the manuscript and revisions, conducted all data analysis, made the figures and performed the proteomics experiments. A.C. and A.M. conducted the failed queen survey, with assistance from H.H. and M.M.G. H.H. and M.M.G. executed the queen shipment temperature tracking. J.M. performed the survival experiments. M.M.G. and J.S.P. performed the drone sperm viability analyses. R.U. contributed the age-matched failed and healthy queens. J.S.P. performed the queen sperm viability measurements across the range of temperatures and measured internal hive temperatures. Grants to D.R.T., J.S.P., M.M.G., A.M. and L.J.F. supported this research. All authors contributed intellectually.
Corresponding authors
Ethics declarations
Competing interests
J.S.P. owns a honey bee consulting business. All other authors have no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–4 and Tables 1 and 2.
Supplementary Data 1
This file contains global LFQ protein expression data and GO term enrichment results for ovaries, spermathecae and semen.
Source data
Source Data Fig. 1
Sperm viability data for failed and healthy queens collected from beekeepers, temperature data for shipments and temperature data for hives.
Source Data Fig. 2
Sperm viability data for temperature-stressed queens and drones.
Rights and permissions
About this article
Cite this article
McAfee, A., Chapman, A., Higo, H. et al. Vulnerability of honey bee queens to heat-induced loss of fertility. Nat Sustain 3, 367–376 (2020). https://doi.org/10.1038/s41893-020-0493-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-020-0493-x
This article is cited by
-
Queen quality, performance, and winter survival of imported and domestic honey bee queen stocks
Scientific Reports (2023)
-
Effects of simulated tropical heat waves during development on the morphological and reproductive traits of Africanized honey bee
Insectes Sociaux (2023)
-
Thermal tolerance role of novel polyamine, caldopentamine, identified in fifth instar Bombyx mori
Amino Acids (2023)
-
Fertility costs of cryptic viral infections in a model social insect
Scientific Reports (2022)
-
Drone honey bees are disproportionately sensitive to abiotic stressors despite expressing high levels of stress response proteins
Communications Biology (2022)