Abstract
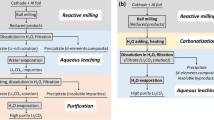
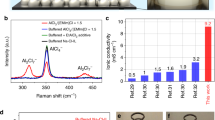
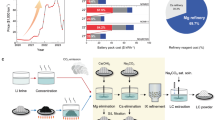
Lithium (Li) is an important resource for the sustainability of socioeconomic systems given its wide use in various industrial applications. The industrial production of Li metals relies on the electrolysis of a mixture consisting of high-purity lithium chloride (LiCl) and potassium chloride. However, the purification of LiCl is expensive and unsustainable, requiring a substantial amount of energy and the use of noxious chemical reagents, so that producing high-purity Li efficiently and sustainably is a challenge. Herein we report a new method of producing high-purity electrolytic Li from low-purity LiCl using solid-state electrolyte. Taking advantage of the high Li-ion selectivity of the solid electrolyte, we directly obtained high-purity metallic Li through the electrolysis of low-purity LiCl. Our new method provides two important advantages over conventional methods: (1) the cost of producing high-purity Li is reduced by using low-purity LiCl from low-grade brine, and the simpler purification process reduces the use of energy and chemical reagents; and (2) the operating temperature of the electrolytic process decreases from 400 °C to 240 °C, leading to an additional reduction in energy use.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Armand, M. & Tarascon, J. M. Building better batteries. Nature 451, 652–657 (2008).
Chu, S., Cui, Y. & Liu, N. The path towards sustainable energy. Nat. Mater. 16, 16–22 (2016).
Dunn, B., Kamath, H. & Tarascon, J. M. Electrical energy storage for the grid: a battery of choices. Science 334, 928–935 (2011).
Larcher, D. & Tarascon, J. M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 7, 19–29 (2015).
Goodenough, J. B. & Park, K. S. The Li-ion rechargeable battery: a perspective. J. Am. Chem. Soc. 135, 1167–1176 (2013).
Lin, D., Liu, Y. & Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 12, 194–206 (2017).
Xu, W. et al. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 7, 513–537 (2014).
Bruce, P. G., Freunberger, S. A., Hardwick, L. J. & Tarascon, J.-M. Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 11, 19–29 (2012).
Gruber, P. W. et al. Global lithium availability. J. Ind. Ecol. 15, 760–775 (2011).
Zhou, Z. Y. et al. Recovery of lithium using tributyl phosphate in methyl isobutyl ketone and FeCl3. Ind. Eng. Chem. Res. 51, 12926–12932 (2012).
Jin, Y. et al. An intermediate temperature garnet-type solid electrolyte-based molten lithium battery for grid energy storage. Nat. Energy 3, 732–738 (2018).
Liu, K. & Wang, C. Garnet-type Li6.4La3Zr1.4Ta0.6O12 thin sheet: fabrication and application. Electrochem. Commun. 48, 147–150 (2014).
Thangadurai, V., Pinzaru, D., Narayanan, S. & Baral, A. K. Fast solid-state Li ion conducting garnet-type structure metal oxides for energy storage. J. Phys. Chem. Lett. 6, 292–299 (2015).
Acknowledgements
This work was supported by the Basic Science Center Program of the National Natural Science Foundation of China (NSFC) under grant no. 51788104 and NSFC projects under grant no. 51661135025. Y.J. acknowledges support from the National Natural Science Foundations of China under grant no. 51807180. Y.L. acknowledges support from the Tsinghua University Initiative Scientific Research Program.
Author information
Authors and Affiliations
Contributions
J.L., H.W. and Y.C. conceived the idea, planned the study, designed the experiment, analysed the data and composed the manuscript. J.L. and Y.J. performed all of the experiments with the assistance of K.L., Y.L., H.Z. and L.Q. H.W. supervised the project. All of the authors reviewed and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–3 and Tables 1–3.
Rights and permissions
About this article
Cite this article
Lang, J., Jin, Y., Liu, K. et al. High-purity electrolytic lithium obtained from low-purity sources using solid electrolyte. Nat Sustain 3, 386–390 (2020). https://doi.org/10.1038/s41893-020-0485-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-020-0485-x
This article is cited by
-
Solar-driven membrane separation for direct lithium extraction from artificial salt-lake brine
Nature Communications (2024)
-
An Electrolysis-Distillation Approach for Producing Potassium Metal
Metallurgical and Materials Transactions B (2024)
-
Lithium metal recycling from spent lithium-ion batteries by cathode overcharging process
Rare Metals (2022)
-
In-situ anodic precipitation process for highly efficient separation of aluminum alloys
Nature Communications (2021)
-
Lithium Extraction from Molten LiOH by Using a Liquid Tin Cathode
Journal of Sustainable Metallurgy (2021)