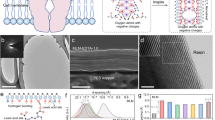
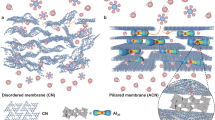
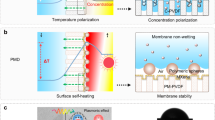
Abstract
Traditional ways of producing drinking water from groundwater, water recycling and water conservation are not sufficient. Seawater desalination would close the gap but the main technology used is thermally driven multi-flash distillation, which is energy consuming and not sustainable. Stacking two-dimensional (2D) nanomaterials into lamellar membranes is a promising technique in the pursuit of both high selectivity and permeance in water desalination. However, 2D membranes tend to swell in water, and increasing their stability in aqueous solution is still challenging. Here, we report non-swelling, MXene membranes prepared by the intercalation of Al3+ ions. Swelling is prevented by strong interactions between Al3+ and oxygen functional groups terminating at the MXene surface. These membranes show excellent non-swelling stability in aqueous solutions up to 400 h and possess high rejection of NaCl (~89.5–99.6%) with fast water fluxes (~1.1–8.5 l m−2 h−1). Such membranes can be easily fabricated by simple filtration and ion-intercalating methods, which holds promise for their scalability.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author on request. Source data for Figs. 1–3 and Extended Data Figs. 1–3 are provided with the paper.
References
Elimelech, M. & Phillip, W. A. The future of seawater desalination: energy, technology, and the environment. Science 333, 712–717 (2011).
Van der Bruggen, B. & Vandecasteele, C. Distillation vs. membrane filtration: overview of process evolutions in seawater desalination. Desalination 143, 207–218 (2002).
Sholl, D. S. & Lively, R. P. Seven chemical separations to change the world. Nature 532, 435–437 (2016).
Van der Bruggen, B., Mänttäri, M. & Nyström, M. Drawbacks of applying nanofiltration and how to avoid them: a review. Sep. Purif. Technol. 63, 251–263 (2008).
Joshi, R. K. et al. Precise and ultrafast molecular sieving through graphene oxide membranes. Science 343, 752–754 (2014).
Mi, B. Graphene oxide membranes for ionic and molecular sieving. Science 343, 740–742 (2014).
Liu, G., Jin, W. & Xu, N. Two-dimensional-material membranes: a new family of high-performance separation membranes. Angew. Chem. Int. Ed. Engl. 55, 13384–13397 (2016).
Han, Y., Xu, Z. & Gao, C. Ultrathin graphene nanofiltration membrane for water purification. Adv. Funct. Mater. 23, 3693–3700 (2013).
Hirunpinyopas, W. et al. Desalination and nanofiltration through functionalized laminar MoS2 membranes. ACS Nano 11, 11082–11090 (2017).
Abraham, J. et al. Tunable sieving of ions using graphene oxide membranes. Nat. Nanotechnol. 12, 546–550 (2017).
Liu, H., Wang, H. & Zhang, X. Facile fabrication of freestanding ultrathin reduced graphene oxide membranes for water purification. Adv. Mater. 27, 249–254 (2015).
Chen, L. et al. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing. Nature 550, 380–383 (2017).
Ren, C. E. et al. Voltage-gated ions sieving through 2D MXene Ti3C2Tx membranes. ACS Appl. Nano Mater. 1, 3644–3652 (2018).
Anasori, B., Lukatskaya, M. R. & Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2, 16098 (2017).
Lukatskaya, M. R. et al. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 341, 1502–1505 (2013).
Naguib, M. et al. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 23, 4248–4253 (2011).
Ding, L. et al. MXene molecular sieving membranes for highly efficient gas separation. Nat. Commun. 9, 155 (2018).
Shen, J. et al. 2D MXene nanofilms with tunable gas transport channels. Adv. Funct. Mater. 28, 1801511 (2018).
Ren, C. E. et al. Charge- and size-selective ion sieving through Ti3C2Tx MXene membranes. J. Phys. Chem. Lett. 6, 4026–4031 (2015).
Ding, L. et al. A two-dimensional lamellar membrane: MXene nanosheet stacks. Angew. Chem. Int. Ed. Engl. 56, 1825–1829 (2017).
Pandey, R. P. et al. Ultrahigh-flux and fouling-resistant membrane based on layered silver/MXene (Ti3C2Tx) nanosheets. J. Mater. Chem. A 6, 3522–3533 (2018).
Wang, J. et al. A regularly channeled lamellar membrane for unparalleled water and organics permeation. Angew. Chem. Int. Ed. Engl. 57, 6814–6818 (2018).
Rasool, K. et al. Antibacterial activity of Ti3C2Tx MXene. ACS Nano 10, 3674–3684 (2016).
Berdiyorov, G. R. & Mahmoud, K. A. Effect of surface termination on ion intercalation selectivity of bilayer Ti3C2T2 (T = F, O and OH) MXene. Appl. Surf. Sci. 416, 725–730 (2017).
Ernst, K. H., Grman, D., Hauert, R. & Holländer, E. Fluorine‐Induced corrosion of aluminium microchip bond pads: An XPS and AES analysis. Surf. Interface Anal. 21, 691–696 (1994).
Halim, J. et al. X-ray photoelectron spectroscopy of select multi-layered transition metal carbides (MXenes). Appl. Surf. Sci. 362, 406–417 (2016).
Ghidiu, M. et al. Ion-exchange and cation solvation reactions in Ti3C2 MXene. Chem. Mater. 28, 3507–3514 (2016).
Muckley, E. S. et al. Multimodality of structural, electrical, and gravimetric responses of intercalated MXenes to water. ACS Nano 11, 11118–11126 (2017).
Lipatov, A. et al. Effect of synthesis on quality, electronic properties and environmental stability of individual monolayer Ti3C2 MXene flakes. Adv. Electron. Mater. 2, 1600255 (2016).
Alhabeb, M. et al. Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2Tx MXene). Chem. Mater. 29, 7633–7644 (2017).
Richards, L. A., Schäfer, A. I., Richards, B. S. & Corry, B. The importance of dehydration in determining ion transport in narrow pores. Small 8, 1701–1709 (2012).
Sahu, S., Di Ventra, M. & Zwolak, M. Dehydration as a universal mechanism for ion selectivity in graphene and other atomically thin pores. Nano Lett. 17, 4719–4724 (2017).
Segall, M. et al. First-principles simulation: ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 14, 2717 (2002).
Perdew, J. P. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Hamann, D. Norm-conserving pseudopotentials. Phys. Rev. Lett. 43, 1494 (1979).
Li, L. et al. Selective gas diffusion in two-dimensional MXene lamellar membranes: insights from molecular dynamics simulations. J. Mater. Chem. A 6, 11734–11742 (2018).
Wang, X. et al. Reversed thermo-switchable molecular sieving membranes composed of two-dimensional metal-organic nanosheets for gas separation. Nat. Commun. 8, 14460 (2017).
Rappé, A. K., Casewit, C. J., Colwell, K., Goddard Iii, W. & Skiff, W. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 114, 10024–10035 (1992).
Kadantsev, E. S., Boyd, P. G., Daff, T. D. & Woo, T. K. Fast and accurate electrostatics in metal organic frameworks with a robust charge equilibration parameterization for high-throughput virtual screening of gas adsorption. J. Phys. Chem. Lett. 4, 3056–3061 (2013).
Berendsen, H., Grigera, J. & Straatsma, T. The missing term in effective pair potentials. J. Phys. Chem. 91, 6269–6271 (1987).
Li, P., Song, L. F. & Merz, K. M. Jr Systematic parameterization of monovalent ions employing the nonbonded model. J. Chem. Theory Comput. 11, 1645–1657 (2015).
Hess, B., Bekker, H., Berendsen, H. J. & Fraaije, J. G. LINCS: a linear constraint solver for molecular simulations. J. Comput. Chem. 18, 1463–1472 (1997).
Feller, S. E., Zhang, Y., Pastor, R. W. & Brooks, B. R. Constant pressure molecular dynamics simulation: the Langevin piston method. J. Chem. Phys. 103, 4613–4621 (1995).
Shirts, M. R., Pitera, J. W., Swope, W. C. & Pande, V. S. Extremely precise free energy calculations of amino acid side chain analogs: comparison of common molecular mechanics force fields for proteins. J. Chem. Phys. 119, 5740–5761 (2003).
Darden, T., York, D. & Pedersen, L. Particle mesh Ewald: an N⋅ log (N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993).
Hess, B., Kutzner, C., Van Der Spoel, D. & Lindahl, E. GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 4, 435–447 (2008).
Berendsen, H. J., van der Spoel, D. & van Drunen, R. GROMACS: a message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 91, 43–56 (1995).
Acknowledgements
We gratefully acknowledge funding from the Natural Science Foundation of China (21536005, 51621001, 21506066, 21606086 and 21861132013), China Postdoctoral Science Foundation (2019TQ0101, 2019M662920), NSFC-DFG (GZ-678), the Natural Science Foundation of the Guangdong Province (2014A030312007) and Guangdong Natural Science Funds for Distinguished Young Scholar (2017A030306002).
Author information
Authors and Affiliations
Contributions
L.D., Y.Wei, H.W. and J.C. conceived the idea and designed the experiments. L.D. synthesized the materials and carried out most of the characterizations. Y.Wu, Z.L. and J.D. helped with some of the characterizations. L.D. contributed to the DFT calculations. L.D., L.L. and Y.L. contributed to the MD simulations. L.D., L.L., Y.Wei, H.W. and J.C. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 XPS spectra of the MXMs.
The XPS spectra of untreated MXM and Al3+-intercalated MXM. a, Survey spectra of untreated MXM and Al3+-intercalated MXM. b, High-resolution XPS spectra of untreated MXM in Al 2p region. There was no peak in Al 2p region, indicating the nonexistence of Al in untreated MXM. c, High-resolution XPS spectra of Al3+-intercalated MXM in Al 2p region. In the Al 2p region, the fitted peak in the Al3+-intercalated MXM located at 74.8 eV binding energy in Al 2p region corresponds to an Al-O bond, indicating that Al ions tend to connect with oxygen groups on surface of MXene nanosheet.
Extended Data Fig. 2 Cycles performance of the Al3+-intercalated MXMs.
Cycles of desalination/drying performance of the Al3+-intercalated MXMs. a, Na+ permeation rate of each cycle. b, XRD results and the d-spacings for the Al3+-intercalated MXMs in the dry state and after cycles of desalination/drying. It can be found that the Na+ permeation rates of Al3+-intercalated MXM almost maintained in the same level during the cycle operation. And the corresponding XRD analysis also shows the stable interlayer spacing of the Al3+-intercalated MXMs even after three cycles of desalination/drying, demonstrating that there was no significant swelling of the membrane once exposed to water again.
Extended Data Fig. 3 Permeation rates of MXMs under different feed concentrations.
Permeation rates of Na+ through Al3+-intercalated MXMs. a, With various feed concentrations (NaCl as the salt solution). b, Salt rejection of 1.1-μm-thick membrane against NaCl concentration. c, Under a real-time changing of feed concentration. The concentration of NaCl in the feed side was first increased from 0.2 M to 2 M with a certain interval of time (5h), and then decreased to 0.2 M, the permeation rates still could come back to the initial state, showing an excellent non-swelling stability of Al3+-intercalated MXMs. It can be seen that the Na+ permeation rates through the Al3+-intercalated MXMs increased linearly rather than exponentially with increasing the salt concentration on the feed side, demonstrating that the Al3+-intercalated MXM could withstand the high driving force generated under a high salt concentration and exhibit good structure stability. Error bars indicate the standard deviation from three different samples.
Supplementary information
Supplementary Information
Supplementary Figs. 1–32, Notes 1–4 and Tables 1–8.
Source data
Source Data Fig. 1
Unmodified SEM and TEM images and experimental source data.
Source Data Fig. 2
Experimental source data.
Source Data Fig. 3
Simulation source data.
Source Data Extended Data Fig. 1
Experimental source data.
Source Data Extended Data Fig. 2
Experimental source data.
Source Data Extended Data Fig. 3
Experimental source data.
Rights and permissions
About this article
Cite this article
Ding, L., Li, L., Liu, Y. et al. Effective ion sieving with Ti3C2Tx MXene membranes for production of drinking water from seawater. Nat Sustain 3, 296–302 (2020). https://doi.org/10.1038/s41893-020-0474-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-020-0474-0
This article is cited by
-
Nanowire-assisted electrochemical perforation of graphene oxide nanosheets for molecular separation
Nature Communications (2024)
-
Formatted PVDF in lamellar composite solid electrolyte for solid-state lithium metal battery
Nano Research (2024)
-
2D MXenes polar catalysts for multi-renewable energy harvesting applications
Nature Communications (2023)
-
Anomalous water molecular gating from atomic-scale graphene capillaries for precise and ultrafast molecular sieving
Nature Communications (2023)
-
Deformation constraints of graphene oxide nanochannels under reverse osmosis
Nature Communications (2023)