Abstract
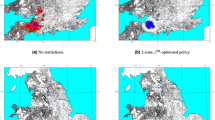
Livestock movements are essential for the economic success of the industry. However, these movements come with the risk of long-range spread of infection, potentially bringing infection to previously disease-free areas where subsequent localized transmission can be devastating. Mechanistic predictive models usually consider controls that minimize the number of livestock affected without considering other costs of an ongoing epidemic. However, it is more appropriate to consider the economic burden, as movement restrictions have major consequences for the economic revenue of farms. Here, using mechanistic models of foot-and-mouth disease, bluetongue virus and bovine tuberculosis in the UK, we compare the economically optimal control strategies for these diseases. We show that for foot-and-mouth disease, the optimal strategy is to ban movements in a small radius around infected farms; the balance between disease control and maintaining ‘business as usual’ varies between regions. For bluetongue virus and bovine tuberculosis, we find that the cost of any movement ban is greater than the epidemiological benefits due to the low within-farm prevalence and slow rate of disease spread. This work suggests that movement controls need to be carefully matched to the epidemiological and economic consequences of the disease, and that optimal movement bans are often of far shorter duration than allowed under existing policy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The raw simulation data used to create Fig. 1 in the main text and all figures in the Supplementary Information are available from corresponding author M.J.T. on request. The authors do not have permission to share the farm-level data for the UK. However, the demographic data that includes farm locations, farm sizes and species composition, as well as the data on livestock movements between farms can be accessed via the RADAR system (e-mail: RADAR@apha.gsi.gov.uk).
Code availability
The code used to create and analyse the raw data and produce the figures are available from the corresponding author on request. The code for the simulation models can be accessed by contacting the following contributing authors: the foot-and-mouth disease model, M.J.T.; the bluetongue virus model, S.B. and the bovine tuberculosis model E.B.P.
References
Gibbens, J. C. et al. Descriptive epidemiology of the 2001 foot-and-mouth disease epidemic in Great Britain: the first five months. Vet. Rec. 149, 729–743 (2001).
Keeling, M. J. et al. Dynamics of the 2001 UK foot and mouth epidemic: stochastic dispersal in a heterogeneous landscape. Science 294, 813–817 (2001).
Ferguson, N. M., Donnelly, C. A. & Anderson, R. M. The foot-and-mouth epidemic in Great Britain: pattern of spread and impact of interventions. Science 292, 1155–1160 (2001).
Saegerman, C., Berkvens, D. & Mellor, P. S. Bluetongue epidemiology in the European Union. Emerging infectious diseases. Natl Cent. Infect. Dis. 14, 539–544 (2008).
Mercier, A. et al. Spread rate of lumpy skin disease in the Balkans, 2015–16. Transbound. Emerg. Dis. 65, 240–243 (2018).
Tasioudi, K. E. et al. Emergence of lumpy skin disease in Greece, 2015. Transbound. Emerg. Dis. 63, 260–265 (2016).
Docuel, V. et al. Epidemiology, molecular virology and diagnostics of Schmallenberg virus, an emerging orthobunyavirus in Europe. Vet. Res. 44, 31 (2013).
Hoffmann, B. et al. Novel orthobunyavirus in cattle, Europe, 2011. Emerg. Infect. Dis. 18, 469–472 (2012).
Green, L. E. & George, T. R. N. Assessment of current knowledge of footrot in sheep with particular reference to Dichelobacter nodosus and implications for elimination or control strategies for sheep in Great Britain. Vet. J. 175, 173–180 (2008).
Baylis, M. & McIntyre, K. M. Scrapie control under new strain. Nature 432, 810–811 (2004).
Schiller, I. et al. Bovine tuberculosis: a review of current and emerging diagnostic techniques in view of their relevance for disease control and eradication. Transbound. Emerg. Dis. 57, 205–220 (2010).
Brooks Pollock, E., Roberts, G. O. & Keeling, M. J. A dynamic model of bovine tuberculosis spread and control in Great Britain. Nature 511, 228–231 (2014).
Garcia-Alvarez, L., Webb, C. R. & Holmes, M. A. A novel field-based approach to validate the use of network models for disease spread between dairy herds. Epidemiol. Infect. 139, 1863–1874 (2011).
Kiss, I. Z., Green, D. M. & Kao, R. R. The effect of network mixing patterns on epidemic dynamics and the efficacy of disease contact tracing. J. Roy. Soc. Interface 5, 791–799 (2008).
Contingency Plan for Exotic Notifiable Diseases of Animals in England (DEFRA, 2017); https://www.gov.uk/government/publications/contingency-plan-for-exotic-notifiable-diseases-of-animals-in-england
Thompson, D. et al. Economic costs of the foot and mouth disease outbreak in the United Kingdom in 2001. Rev. Sci. Tech. 21, 675–687 (2002).
Elbers, A. et al. Field observations during the bluetongue serotype 8 epidemic in 2006: I. Detection of first outbreaks and clinical signs in sheep and cattle in Belgium, France and the Netherlands. Prev. Vet. Med. 87, 21–30 (2008).
Krebs, J. R. et al. Bovine Tuberculosis in Cattle and Badgers (Ministry of Agriculture, Fisheries and Food, 1997); http://www.bovinetb.info/docs/krebs.pdf
Donnelly, C. A. & Nouvellet, P. The contribution of badgers to confirmed tuberculosis in cattle in high-incidence areas in England. PLoS Curr. 1, 097a904d3f3619db2fe78d24bc776098 (2013).
Inamura M., Rushton J. & Antón J. Risk Management of Outbreaks of Livestock Diseases OECD Food, Agriculture and Fisheries Papers No. 91 (OECD, 2015).
Yang, P. C., Chu, R. M., Chung, W. B. & Sung, H. T. Epidemiological characteristics and financial costs of the 1997 foot-and-mouth disease epidemic in Taiwan. Vet. Rec. 145, 731–734 (1999).
Bicknell, K. B., Wilen, J. E. & Howitt, R. E. Public policy and private incentives for livestock disease control. Aust. J. Agric. Resour. Econ. 43, 501–521 (2002).
Epanchin-Niell, R. S. & Wilen, J. E. Optimal spatial control of biological invasions. J. Environ. Econ. Manag. 63, 260–270 (2012).
Olson, L. J. & Roy, S. Controlling a biological invasion: a non-classical dynamic economic model. Econ. Theory 36, 453–469 (2008).
Probert, W. J. M. et al. Real-time decision-making during emergency disease outbreaks. PLoS Comput. Biol. 14, e1006202 (2018).
Feng, S., Patton, M. & Davis, J. Market impact of foot-and-mouth disease control strategies: a UK case study. Front. Vet. Sci. 4, 129 (2017).
Rich, K. M. & Winter-Nelson, A. An integrated epidemiological-economic analysis of foot and mouth disease: applications to the southern cone of South America. Am. J. Agric. Econ. 89, 682–697 (2007).
Longworth, N., Mourits, M. C. & Saatkamp, H. W. Economic analysis of HPAI control in the Netherlands II: comparison of control strategies. Transbound. Emerg. Dis. 61, 217–232 (2014).
Rich, K. M. & Wanyoike, F. An assessment of the regional and national socio-economic impacts of the 2007 Rift Valley fever outbreak in Kenya. Am. J. Trop. Med. Hyg. 83(2 Suppl), 52–57 (2017).
Tildesley, M. J. et al. Optimal reactive vaccination strategies for a foot-and-mouth outbreak in the UK. Nature 440, 83–86 (2016).
Brand, S. P. C. & Keeling, M. J. The impact of temperature changes on vector-borne disease transmission: Culicoides midges and bluetongue virus. J. R. Soc. Interface 14, 20160481 (2017).
Woolhouse, M. et al. Epidemiology. Foot-and-mouth disease under control in the UK. Nature 411, 258–259 (2001).
Sumner, T., Orton, R. J., Green, D. M., Kao, R. R. & Gubbins, S. Quantifying the roles of host movement and vector dispersal in the transmission of vector-borne diseases of livestock. PLoS Comput. Biol. 13, e1005470–22 (2017).
Casey-Bryars, M. et al. Waves of endemic foot-and-mouth disease in eastern Africa suggest feasibility of proactive vaccination approaches. Nat. Ecol. Evol. 2, 1449–1457 (2018).
Anderson, I. Foot & Mouth Disease 2001: Lessons to be Learned Inquiry Report (The Stationary Office, 2002).
Gunn, G. et al. Assessing the Economic Impact of Different Bluetongue Virus (BTV) Incursion Scenarios in Scotland (Scottish Government, 2008).
Measures to Address Bovine TB in Badgers (DEFRA, 2011); https://www.gov.uk/government/publications/measures-to-address-bovine-tuberculosis-in-badgers-impact-assessment
Diggle, P. Spatio-temporal point processes, partial likelihood, foot and mouth disease. Stat. Methods Med. Res. 15, 325–336 (2006).
Tildesley, M. J. et al. Accuracy of models for the 2001 UK foot-and-mouth epidemic. Proc. R. Soc. B 275, 1459–1468 (2008).
Deardon, R. et al. Inference for individual-level models of infectious diseases in large populations. Stat. Sin. 20, 239–261 (2010).
Jewell, C. P., Kypraios, T., Neal, P. & Roberts, G. O. Bayesian analysis for emerging infectious diseases. Bayesian Anal. 4, 465–496 (2009).
Kao, R. R., Danon, L., Green, D. M. & Kiss, I. Z. Demographic structure and pathogen dynamics on the network of livestock movements in Great Britain. Proc. R. Soc. B 273, 1999–2007 (2006).
Green, D. M., Kiss, I. Z. & Kao, R. R. Modelling the initial spread of foot-and-mouth disease through animal movements. Proc. R. Soc. B 273, 2729–2735 (2006).
Dawson, P. M., Werkman, M., Brooks Pollock, E. & Tildesley, M. J. Epidemic predictions in an imperfect world: modelling disease spread with partial data. Proc. R. Soc. B 282, 20150205 (2015).
Szmaragd, C. et al. A modeling framework to describe the transmission of bluetongue virus within and between farms in Great Britain. PLoS ONE 4, e7741 (2009).
Turner, J., Bowers, R. G. & Baylis, M. Modelling bluetongue virus transmission between farms using animal and vector movements. Sci. Rep. 2, 319 (2012).
Græsbøll, K., Bødker, R., Enøe, C. & Christiansen, L. E. Simulating spread of bluetongue virus by flying vectors between hosts on pasture. Sci. Rep. 2, 863 (2012).
Gubbins, S., Carpenter, S., Baylis, M., Wood, J. L. N. & Mellor, P. S. Assessing the risk of bluetongue to UK livestock: uncertainty and sensitivity analyses of a temperature-dependent model for the basic reproduction number. J. R. Soc. Interface 5, 363–371 (2008).
United Kingdom Climate Projections (UKCP09) (Met Office, 2009); https://www.metoffice.gov.uk/climatechange/science/monitoring/ukcp09/
Carpenter, S. et al. Temperature dependence of the extrinsic incubation period of orbiviruses in culicoides biting midges. PLoS ONE 6, e27987 (2011).
Sanders, C. J. et al. Influence of season and meteorological parameters on flight activity of Culicoides biting midges. J. Appl. Ecol. 48, 1355–1364 (2011).
Kettle, D. S. The bionomics and control of Culicoides and Leptoconops (Diptera, Ceratopogonidae = Heleidae). Annu. Rev. Entomol. 7, 401–418 (1962).
Mellor, P. S., Boorman, J. & Baylis, M. Culicoides biting midges: their role as arbovirus vectors. Annu. Rev. Entomol. 45, 307–340 (2000).
UK Bluetongue Control Strategy (DEFRA, 2008); https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/343741/bluetongue-control-strategy081201.pdf
Karolemeas, K. et al. Estimation of the relative sensitivity of the comparative tuberculin skin test in tuberculous cattle herds subjected to depopulation. PLoS ONE 7, e43217 (2012).
Acknowledgements
M.J.T. and M.J.K. acknowledge support from the Biotechnology and Biological Sciences Research Council (grant number BB/K010972/4). We thank M. Ferrari and W. Probert for useful discussions regarding this manuscript.
Author information
Authors and Affiliations
Contributions
M.J.T., S.B., E.B.P. and M.W. carried out simulations using the three livestock disease models. N.V.B. developed the Shiny app for visualization of sensitivity analysis. M.J.K. and M.J.T. analysed the outputs of the simulation models. M.J.K. led the sensitivity analysis of the modelling results and provided intellectual expertise on all three livestock disease models. All authors contributed to the writing of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary notes, Supplementary Tables 1–4, Supplementary Figs. 1–4 and Supplementary references 1–37.
Rights and permissions
About this article
Cite this article
Tildesley, M.J., Brand, S., Brooks Pollock, E. et al. The role of movement restrictions in limiting the economic impact of livestock infections. Nat Sustain 2, 834–840 (2019). https://doi.org/10.1038/s41893-019-0356-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-019-0356-5
This article is cited by
-
Optimal control of animal diseases
Nature Sustainability (2019)