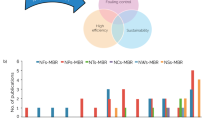
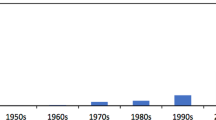
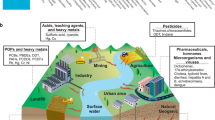
Abstract
Sustainable provision of safe, clean and adequate water supply is a global challenge. Water treatment and desalination technologies remain chemically and energy intensive, ineffective in removing key trace contaminants, and poorly suited to deployment in decentralized (distributed) water treatment systems globally. Several recent efforts have sought to leverage the reactive and tunable properties of nanomaterials to address these technological shortcomings. This Review assesses the potential applications of nanomaterials in advancing sustainable water treatment systems and proposes ways to evaluate the environmental risks and social acceptance of nanotechnology-enabled water treatment processes. Future areas of research necessary to realize safe deployment of promising nanomaterial applications are also identified.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Shannon, M. A. et al. Science and technology for water purification in the coming decades. Nature 452, 301–310 (2008).
Becker, G. S. in Human Capital: A Theoretical and Empirical Analysis with Special Reference to Education 3rd edn, 15–28 (Univ. Chicago Press, 1994).
Costanza, R. & Daly, H. E. Natural capital and sustainable development. Conserv. Biol. 6, 37–46 (1992).
Tal, A. Seeking sustainability: Israel’s evolving water management strategy. Science 313, 1081–1084 (2006).
Mauter, M. S. & Elimelech, M. Environmental applications of carbon-based nanomaterials. Environ. Sci. Technol. 42, 5843–5859 (2008).
Matson, P., Clark, W. C. & Andersson, K. Pursuing Sustainability: A Guide to the Science and Practice (Princeton Univ. Press, 2016).
Progress on Drinking Water, Sanitation and Hygiene: 2017 Update and SDG Baselines (World Health Organization (WHO) and United Nations Children’s Fund (UNICEF), 2017).
US Environmental Protection Agency. Drinking water contaminant candidate list. Fed. Reg. 81, 81099–81114 (2016).
Li, Q. et al. Antimicrobial nanomaterials for water disinfection and microbial control: potential applications and implications. Water Res .42, 4591–4602 (2008).
Bhadra, C. M. et al. Antibacterial titanium nano-patterned arrays inspired by dragonfly wings. Sci. Rep. 5, 16817 (2015).
Ivanova, E. P. et al. Bactericidal activity of black silicon. Nat. Commun. 4, 2838 (2013).
Gazit, E. Self-assembled peptide nanostructures: the design of molecular building blocks and their technological utilization. Chem. Soc. Rev. 36, 1263–1269 (2007).
Kang, S., Pinault, M., Pfefferle, L. D. & Elimelech, M. Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir 23, 8670–8673 (2007).
Wang, Y. et al. High-performance capacitive deionization disinfection of water with graphene oxide-graft-quaternized chitosan nanohybrid electrode coating. ACS Nano 9, 10142–10157 (2015).
Cho, M., Chung, H., Choi, W. & Yoon, J. Different inactivation behaviors of MS-2 phage and Escherichia coli in TiO2 photocatalytic disinfection. Appl. Environ. Microbiol. 71, 270–275 (2005).
Liu, C. et al. Rapid water disinfection using vertically aligned MoS2 nanofilms and visible light. Nat. Nanotechnol. 11, 1098–1104 (2016).
Zhang, D. Q., Li, G. S. & Yu, J. C. Inorganic materials for photocatalytic water disinfection. J. Mater. Chem. 20, 4529–4536 (2010).
Xiu, Z. M., Zhang, Q. B., Puppala, H. L., Colvin, V. L. & Alvarez, P. J. J. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 12, 4271–4275 (2012).
Propato, M. & Uber, J. G. Vulnerability of water distribution systems to pathogen intrusion: How effective is a disinfectant residual? Environ. Sci. Technol. 38, 3713–3722 (2004).
Raghupathi, K. R., Koodali, R. T. & Manna, A. C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 27, 4020–4028 (2011).
Yu, J. C. et al. Efficient visible-light-induced photocatalytic disinfection on sulfur-doped nanocrystalline titania. Environ. Sci. Technol. 39, 1175–1179 (2005).
Lee, I. et al. Photochemical and antimicrobial properties of novel C60 derivatives in aqueous systems. Environ. Sci. Technol. 43, 6604–6610 (2009).
Cho, M., Snow, S. D., Hughes, J. B. & Kim, J. H. Escherichia coli inactivation by UVC-irradiated C60: kinetics and mechanisms. Environ. Sci. Technol. 45, 9627–9633 (2011).
Liu, S. et al. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress. ACS Nano 5, 6971–6980 (2011).
Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 415, 389–395 (2002).
Lu, X. et al. Enhanced antibacterial activity through the controlled alignment of graphene oxide nanosheets. Proc. Natl. Acad. Sci. USA 114, E9793–E9801 (2017).
Snow, S. D., Park, K. & Kim, J. H. Cationic fullerene aggregates with unprecedented virus photoinactivation efficiencies in water. Environ. Sci. Tech. Lett. 1, 290–294 (2014).
Schwarzenbach, R. P. et al. The challenge of micropollutants in aquatic systems. Science 313, 1072 (2006).
Huber, M. M., Canonica, S., Park, G. Y. & von Gunten, U. Oxidation of pharmaceuticals during ozonation and advanced oxidation processes. Environ. Sci. Technol. 37, 1016–1024 (2003).
Ashby, M. F., Ferreira, P. J. & Schodek, D. L. in Nanomaterials, Nanotechnologies and Design 177–197 (Butterworth-Heinemann, 2009).
Chiu, C. Y. et al. Platinum nanocrystals selectively shaped using facet-specific peptide sequences. Nat. Chem. 3, 393–399 (2011).
Waychunas, G. A., Kim, C. S. & Banfield, J. F. Nanoparticulate iron oxide minerals in soils and sediments: unique properties and contaminant scavenging mechanisms. J. Nanopart. Res. 7, 409–433 (2005).
Madden, A. S., Hochella, M. F. & Luxton, T. P. Insights for size-dependent reactivity of hematite nanomineral surfaces through Cu2+ sorption. Geochim. Cosmochim. Acta 70, 4095–4104 (2006).
Cai, W. Q., Yu, J. G., Cheng, B., Su, B. L. & Jaroniec, M. Synthesis of boehmite hollow core/shell and hollow microspheres via sodium tartrate-mediated phase transformation and their enhanced adsorption performance in water treatment. J. Phys. Chem. C. 113, 14739–14746 (2009).
Pan, B. & Xing, B. Adsorption mechanisms of organic chemicals on carbon nanotubes. Environ. Sci. Technol. 42, 9005–9013 (2008).
Wang, J., Chen, Z. & Chen, B. Adsorption of polycyclic aromatic hydrocarbons by graphene and graphene oxide nanosheets. Environ. Sci. Technol. 48, 4817–4825 (2014).
Hua, M. et al. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 211–212, 317–331 (2012).
Lounsbury, A. W., Yamani, J. S., Johnston, C. P., Larese-Casanova, P. & Zimmerman, J. B. The role of counter ions in nano-hematite synthesis: Implications for surface area and selenium adsorption capacity. J. Hazard. Mater. 310, 117–124 (2016).
Vuković, G. D. et al. Removal of cadmium from aqueous solutions by oxidized and ethylenediamine-functionalized multi-walled carbon nanotubes. Chem. Eng. J. 157, 238–248 (2010).
Yuan, Q. et al. Facet-dependent selective adsorption of Mn-doped α-Fe2O3 nanocrystals toward heavy-metal ions. Chem. Mater. 29, 10198–10205 (2017).
Eckelman, M. J., Mauter, M. S., Isaacs, J. A. & Elimelech, M. New perspectives on nanomaterial aquatic ecotoxicity: production impacts exceed direct exposure impacts for carbon nanotoubes. Environ. Sci. Technol. 46, 2902–2910 (2012).
Apul, O. G., Wang, Q., Zhou, Y. & Karanfil, T. Adsorption of aromatic organic contaminants by graphene nanosheets: Comparison with carbon nanotubes and activated carbon. Water Res .47, 1648–1654 (2013).
Beless, B., Rifai, H. S. & Rodrigues, D. F. Efficacy of carbonaceous materials for sorbing polychlorinated biphenyls from aqueous solution. Environ. Sci. Technol. 48, 10372–10379 (2014).
Cooper, A. M., Hristovski, K. D., Möller, T., Westerhoff, P. & Sylvester, P. The effect of carbon type on arsenic and trichloroethylene removal capabilities of iron (hydr)oxide nanoparticle-impregnated granulated activated carbons. J. Hazard. Mater. 183, 381–388 (2010).
Farrell, J. W. et al. Arsenic removal by nanoscale magnetite in Guanajuato, Mexico. Environ. Eng. Sci. 31, 393–402 (2014).
Savage, N. & Diallo, M. S. Nanomaterials and water purification: opportunities and challenges. J. Nanopart. Res. 7, 331–342 (2005).
Lee, J. et al. C60 aminofullerene immobilized on silica as a visible-light-activated photocatalyst. Environ. Sci. Technol. 44, 9488–9495 (2010).
Oulton, R. et al. Hydroxyl radical formation during ozonation of multiwalled carbon nanotubes: performance optimization and demonstration of a reactive CNT filter. Environ. Sci. Technol. 49, 3687–3697 (2015).
Asahi, R., Morikawa, T., Ohwaki, T., Aoki, K. & Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 293, 269–271 (2001).
El-Sheikh, S. M. et al. Tailored synthesis of anatase–brookite heterojunction photocatalysts for degradation of cylindrospermopsin under UV–Vis light. Chem. Eng. J. 310, 428–436 (2017).
Liu, S., Yu, J. & Jaroniec, M. Tunable photocatalytic selectivity of hollow TiO2 microspheres composed of anatase polyhedra with exposed {001} facets. J. Am. Chem. Soc. 132, 11914–11916 (2010).
Su, J. et al. Highly efficient and recyclable triple-shelled Ag@Fe3O4@SiO2@TiO2 photocatalysts for degradation of organic pollutants and reduction of hexavalent chromium ions. Nanoscale .6, 5181–5192 (2014).
Horovitz, I. et al. Carbamazepine degradation using a N-doped TiO2 coated photocatalytic membrane reactor: Influence of physical parameters. J. Hazard. Mater. 310, 98–107 (2016).
Benotti, M. J., Stanford, B. D., Wert, E. C. & Snyder, S. A. Evaluation of a photocatalytic reactor membrane pilot system for the removal of pharmaceuticals and endocrine disrupting compounds from water. Water Res .43, 1513–1522 (2009).
Cates, E. L. Photocatalytic water treatment: So where are we going with this? Environ. Sci. Technol. 51, 757–758 (2017).
Roco, M. C. The long view of nanotechnology development: the National Nanotechnology Initiative at 10 years. J. Nanopart. Res 13, 427–445 (2011).
Elimelech, M. & Phillip, W. A. The future of seawater desalination: energy, technology, and the environment. Science 333, 712–717 (2011).
Werber, J. R., Osuji, C. O. & Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 1, 16018 (2016).
Werber, J. R., Deshmukh, A. & Elimelech, M. The critical need for increased selectivity, not increased water permeability, for desalination membranes. Environ. Sci. Tech. Lett. 3, 112–120 (2016).
Jeong, B. H. et al. Interfacial polymerization of thin film nanocomposites: A new concept for reverse osmosis membranes. J. Membr. Sci. 294, 1–7 (2007).
Liu, Y. & Chen, X. High permeability and salt rejection reverse osmosis by a zeolite nano-membrane. Phys. Chem. Chem. Phys. 15, 6817–6824 (2013).
Joshi, R. K. et al. Precise and ultrafast molecular sieving through graphene oxide membranes. Science 343, 752–754 (2014).
Majumder, M., Chopra, N., Andrews, R. & Hinds, B. J. Nanoscale hydrodynamics: enhanced flow in carbon nanotubes. Nature 438, 44 (2005).
Holt, J. K. et al. Fast mass transport through sub-2-nanometer carbon nanotubes. Science 312, 1034–1037 (2006).
Corry, B. Designing carbon nanotube membranes for efficient water desalination. J. Phys. Chem. B .112, 1427–1434 (2008).
Mi, B. Graphene oxide membranes for ionic and molecular sieving. Science 343, 740–742 (2014).
Amadei, C. A. & Vecitis, C. D. How to increase the signal-to-noise ratio of graphene oxide membrane research. J. Phys. Chem. Lett. 7, 3791–3797 (2016).
Wei, N., Peng, X. & Xu, Z. Breakdown of fast water transport in graphene oxides. Phys. Rev. E. 89, 012113 (2014).
Zhou, X. et al. Self-assembling subnanometer pores with unusual mass-transport properties. Nat. Commun. 3, 949 (2012).
Licsandru, E. et al. Salt-excluding artificial water channels exhibiting enhanced dipolar water and proton translocation. J. Am. Chem. Soc. 138, 5403–5409 (2016).
Zhou, M. et al. New type of membrane material for water desalination based on a cross-linked bicontinuous cubic lyotropic liquid crystal assembly. J. Am. Chem. Soc. 129, 9574–9575 (2007).
Shen, Y. X. et al. Highly permeable artificial water channels that can self-assemble into two-dimensional arrays. Proc. Natl Acad. Sci. USA 112, 9810–9815 (2015).
Perreault, F., Tousley, M. E. & Elimelech, M. Thin-film composite polyamide membranes functionalized with biocidal graphene oxide nanosheets. Environ. Sci. Tech. Lett. 1, 71–76 (2014).
Tiraferri, A., Vecitis, C. D. & Elimelech, M. Covalent binding of single-walled carbon nanotubes to polyamide membranes for antimicrobial surface properties. ACS Appl. Mater. Inter. 3, 2869–2877 (2011).
Liu, C., Lee, J., Small, C., Ma, J. & Elimelech, M. Comparison of organic fouling resistance of thin-film composite membranes modified by hydrophilic silica nanoparticles and zwitterionic polymer brushes. J. Membr. Sci. 544, 135–142 (2017).
Oberdörster, G., Oberdörster, E. & Oberdörster, J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 113, 823–839 (2005).
Maynard, A. D., Warheit, D. B. & Philbert, M. A. The new toxicology of sophisticated materials: nanotoxicology and beyond. Toxicol. Sci. 120(Suppl.), S109–129 (2011).
Bondarenko, O. et al. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Arch. Toxicol. 87, 1181–1200 (2013).
Nel, A. E. et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 8, 543–557 (2009).
Gilbertson, L. M. et al. Shape-dependent surface reactivity and antimicrobial activity of nano-cupric oxide. Environ. Sci. Technol. 50, 3975–3984 (2016).
Perreault, F., de Faria, A. F., Nejati, S. & Elimelech, M. Antimicrobial properties of graphene oxide nanosheets: Why size matters. ACS Nano 9, 7226–7236 (2015).
Pasquini, L. M., Hashmi, S. M., Sommer, T. J., Elimelech, M. & Zimmerman, J. B. Impact of surface functionalization on bacterial cytotoxicity of single-walled carbon nanotubes. Environ. Sci. Technol. 46, 6297–6305 (2012).
Hull, M., Kennedy, A. J., Detzel, C., Vikesland, P. & Chappell, M. A. Moving beyond mass: the unmet need to consider dose metrics in environmental nanotoxicology studies. Environ. Sci. Technol. 46, 10881–10882 (2012).
Petersen, E. J. et al. Adapting OECD aquatic toxicity tests for use with manufactured nanomaterials: Key issues and consensus recommendations. Environ. Sci. Technol. 49, 9532–9547 (2015).
Nelson, B. C. et al. NIST gold nanoparticle reference materials do not induce oxidative DNA damage. Nanotoxicology 7, 21–29 (2013).
Taurozzi, J. S., Hackley, V. A. & Wiesner, M. R. A standardised approach for the dispersion of titanium dioxide nanoparticles in biological media. Nanotoxicology 7, 389–401 (2013).
DeLoid, G. et al. Estimating the effective density of engineered nanomaterials for in vitro dosimetry. Nat. Commun. 5, 3514 (2014).
DeLoid, G. M., Cohen, J. M., Pyrgiotakis, G. & Demokritou, P. Preparation, characterization, and in vitro dosimetry of dispersed, engineered nanomaterials. Nat. Protoc. 12, 355–371 (2017).
Holden, P. A. et al. Considerations of environmentally relevant test conditions for improved evaluation of ecological hazards of engineered nanomaterials. Environ. Sci. Technol. 50, 6124–6145 (2016).
Levard, C., Hotze, E. M., Lowry, G. V. & Brown, G. E. Jr. Environmental transformations of silver nanoparticles: Impact on stability and toxicity. Environ. Sci. Technol. 46, 6900–6914 (2012).
Lowry, G. V., Gregory, K. B., Apte, S. C. & Lead, J. R. Transformations of nanomaterials in the environment. Environ. Sci. Technol. 46, 6893–6899 (2012).
Dale, A. L. et al. Modeling nanomaterial environmental fate in aquatic systems. Environ. Sci. Technol. 49, 2587–2593 (2015).
Keller, A. A., McFerran, S., Lazareva, A. & Suh, S. Global life cycle releases of engineered nanomaterials. J. Nanopart. Res. 15, 1692–1709 (2013).
Sun, T. Y. et al. Probabilistic modelling of engineered nanomaterial emissions to the environment: a spatio-temporal approach. Environ. Sci. Nano 2, 340–351 (2015).
Coll, C. et al. Probabilistic environmental risk assessment of five nanomaterials (nano-TiO2, nano-Ag, nano-ZnO, CNT, and fullerenes). Nanotoxicology 10, 436–444 (2016).
Westerhoff, P., Alvarez, P. J. J., Li, Q., Gardea-Torresdey, J. & Zimmerman, J. Overcoming implementation barriers for nanotechnology in drinking water treatment. Environ. Sci. Nano 3, 1241–1253 (2016).
Ma, R. et al. Fate of zinc oxide and silver nanoparticles in a pilot wastewater treatment plant and in processed biosolids. Environ. Sci. Technol. 48, 104–112 (2014).
Conway, J. R., Adeleye, A. S., Gardea-Torresdey, J. & Keller, A. A. Aggregation, dissolution, and transformation of copper nanoparticles in natural waters. Environ. Sci. Technol. 49, 2749–2756 (2015).
Grillo, R., Rosa, A. H. & Fraceto, L. F. Engineered nanoparticles and organic matter: A review of the state-of-the-art. Chemosphere 119, 608–619 (2015).
Garg, S., Rong, H., Miller, C. J. & Waite, T. D. Oxidative dissolution of silver nanoparticles by chlorine: implications to silver nanoparticle fate and toxicity. Environ. Sci. Technol. 50, 3890–3896 (2016).
Du, T. et al. Photochlorination-induced transformation of graphene oxide: Mechanism and environmental fate. Water Res 124, 372–380 (2017).
Notter, D. A., Mitrano, D. M. & Nowack, B. Are nanosized or dissolved metals more toxic in the environment? A meta-analysis. Environ. Toxicol. Chem. 33, 2733–2739 (2014).
van der Zande, M. et al. Distribution, elimination, and toxicity of silver nanoparticles and silver ions in rats after 28-day oral exposure. ACS Nano 6, 7427–7442 (2012).
Buesen, R. et al. Effects of SiO2, ZrO2, and BaSO4 nanomaterials with or without surface functionalization upon 28-day oral exposure to rats. Arch. Toxicol. 88, 1881–1906 (2014).
Alison, A., Stuart, A., Alan, P. & Clare, W. The framing of nanotechnologies in the British newspaper press. Sci. Commun. 27, 200–220 (2005).
Cobb, M. D. & Macoubrie, J. Public perceptions about nanotechnology: Risks, benefits and trust. J. Nanopart. Res. 6, 395–405 (2004).
Mitrano, D. M. & Nowack, B. The need for a life-cycle based aging paradigm for nanomaterials: importance of real-world test systems to identify realistic particle transformations. Nanotechnology 28, 072001 (2017).
Stacey, H. et al. Measuring nanomaterial release from carbon nanotube composites: Review of the state of the science. J. Phys. Conf. Ser. 617, 012026 (2015).
Dahlben, L. J., Eckelman, M. J., Hakimian, A., Somu, S. & Isaacs, J. A. Environmental life cycle assessment of a carbon nanotube-enabled semiconductor device. Environ. Sci. Technol. 47, 8471–8478 (2013).
Anderson, P., Wiener, J., Graham, J. & Weiner, J. Risk versus Risk: Tradeoffs in Protecting Health and the Environment (Harvard Univ. Press, Cambridge, 1995).
Eckelman, M. J., Mauter, M. S., Isaacs, J. A. & Elimelech, M. New perspectives on nanomaterial aquatic ecotoxicity: production impacts exceed direct exposure impacts for carbon nanotoubes. Environ. Sci. Technol. 46, 2902–2910 (2012).
Gingerich, D. B. & Mauter, M. S. Air emissions damages from municipal drinking water treatment under current and proposed regulatory standards. Environ. Sci. Technol. 51, 10299–10306 (2017).
Hering, J. G., Waite, T. D., Luthy, R. G., Drewes, Jr. E. & Sedlak, D. L. A changing framework for urban water systems. Environ. Sci. Technol. 47, 10721–10726 (2013).
Kiparsky, M., Sedlak, D. L., Thompson, B. H. Jr. & Truffer, B. The innovation deficit in urban water: The need for an integrated perspective on institutions, organizations, and technology. Environ. Eng. Sci. 30, 395–408 (2013).
Brame, J., Li, Q. L. & Alvarez, P. J. J. Nanotechnology-enabled water treatment and reuse: emerging opportunities and challenges for developing countries. Trends Food Sci. Tech. 22, 618–624 (2011).
Ren, D., Colosi, L. M. & Smith, J. A. Evaluating the sustainability of ceramic filters for point-of-use drinking water treatment. Environ. Sci. Technol. 47, 11206–11213 (2013).
Saidi, T. & Zeiss, R. Investigating promises of nanotechnology for development: A case study of the travelling of smart nano water filter in Zimbabwe. Technol. Soc. 46, 40–48 (2016).
Novak, P. J. et al. Innovation promoted by regulatory flexibility. Environ. Sci. Technol. 49, 13908–13909 (2015).
Coglianese, C. & Lazer, D. Management‐based regulation: Prescribing private management to achieve public goals. Law Soc. Rev. 37, 691–730 (2003).
Qu, X., Brame, J., Li, Q. & Alvarez, P. J. J. Nanotechnology for a safe and sustainable water supply: enabling integrated water treatment and reuse. Acc. Chem. Res. 46, 834–843 (2013).
Kraus, N., Malmfors, T. & Slovic, P. Intuitive toxicology — expert and lay judgments of chemical risks. Risk Anal. 12, 215–232 (1992).
Scheufele, D. A. et al. Scientists worry about some risks more than the public. Nat. Nanotechnol. 2, 732–734 (2007).
van Dijk, H., Fischer, A. R. H., Marvin, H. J. P. & van Trijp, H. C. M. Determinants of stakeholders’ attitudes towards a new technology: nanotechnology applications for food, water, energy and medicine. J. Risk Res. 20, 277–298 (2015).
Loeb, S., Li, C. & Kim, J.-H. Solar photothermal disinfection using broadband-light absorbing gold nanoparticles and carbon black. Environ. Sci. Technol. 52, 205–213 (2018).
Hutchison, J. E. The road to sustainable nanotechnology: Challenges, progress and opportunities. ACS Sustain. Chem. Eng. 4, 5907–5914 (2016).
Acknowledgements
We acknowledge the support received from the US National Science Foundation (NSF) through the Nanosystems Engineering Research Center for Nanotechnology Enabled Water Treatment (Grant EEC-1449500).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mauter, M.S., Zucker, I., Perreault, F. et al. The role of nanotechnology in tackling global water challenges. Nat Sustain 1, 166–175 (2018). https://doi.org/10.1038/s41893-018-0046-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-018-0046-8
This article is cited by
-
Effects of nano-TiO2/Fe3O4 addition on soil phosphorus fractions, microbial characteristics, and plant growth
Journal of Soils and Sediments (2024)
-
Smart dynamic hybrid membranes with self-cleaning capability
Nature Communications (2023)
-
Mimicking reductive dehalogenases for efficient electrocatalytic water dechlorination
Nature Communications (2023)
-
Hydrodynamic tearing of bacteria on nanotips for sustainable water disinfection
Nature Communications (2023)
-
Towards the realisation of high permi-selective MoS2 membrane for water desalination
npj Clean Water (2023)