Abstract
Substantial interest and investment in clinical artificial intelligence (AI) research has not resulted in widespread translation to deployed AI solutions. Current attention has focused on bias and explainability in AI algorithm development, external validity and model generalisability, and lack of equity and representation in existing data. While of great importance, these considerations also reflect a model-centric approach seen in published clinical AI research, which focuses on optimising architecture and performance of an AI model on best available datasets. However, even robustly built models using state-of-the-art algorithms may fail once tested in realistic environments due to unpredictability of real-world conditions, out-of-dataset scenarios, characteristics of deployment infrastructure, and lack of added value to clinical workflows relative to cost and potential clinical risks. In this perspective, we define a vertically integrated approach to AI development that incorporates early, cross-disciplinary, consideration of impact evaluation, data lifecycles, and AI production, and explore its implementation in two contrasting AI development pipelines: a scalable “AI factory” (Mayo Clinic, Rochester, United States), and an end-to-end cervical cancer screening platform for resource poor settings (Paps AI, Mbarara, Uganda). We provide practical recommendations for implementers, and discuss future challenges and novel approaches (including a decentralised federated architecture being developed in the NHS (AI4VBH, London, UK)). Growth in global clinical AI research continues unabated, and introduction of vertically integrated teams and development practices can increase the translational potential of future clinical AI projects.
Similar content being viewed by others
Introduction
Multiple indicators over the past five years demonstrate accelerating interest in the application of artificial intelligence (AI) to human health, including exponentially increasing published research since 20161, increasing healthcare provider interest in AI solutions2,3, soaring investment into AI startups4,5, and year-on-year increases in regulatory approvals6,7. In contrast, widespread translation of AI research into implementation remains conspicuously absent, particularly when considering AI for clinical decision-making, diagnosis, or prediction8. For example, while high-profile studies demonstrate superiority of AI-assisted cancer detection compared to clinicians9, there has been failure to replicate accuracy in larger studies, with limited prospective, real-world validation and poor potential for clinical utility10.
Most clinical AI research is conducted on existing, retrospective datasets11,12,13, where focus is on improving algorithm performance for given internal and external datasets, referred to as a ‘model-centric’ approach14. Research waste, in the form of algorithms that will never see clinical utilisation, continues to increase15,16,17. Unrepresentative data and model bias contribute to these failings18,19, and the push for equitable data accumulation and incremental architectural gains are important for progressing AI as a whole20. However, less consideration is given to real-world factors that maintain importance throughout any AI development pathway, including the real-time data lifecycles that support predictions, heterogeneous software and hardware infrastructure that host AI models, and quantification of impact on patients and clinical workflows. Significance of these factors can be seen in failures of previous real-world evaluations of state-of-the-art algorithms, that have been unable to achieve anticipated performance due to infrastructural and data problems21, or lack of added value within everyday workflows22,23.
In contrast, the use of AI in non-healthcare enterprises has achieved greater success, demonstrating clear return-on-investment24,25. Clearly, intricacies and risks inherent to patient data are not comparable to non-healthcare sectors, but lessons can be taken from differing approaches to AI, focussing on value generation, cross-disciplinary collaboration, and a holistic approach to practicalities external to algorithm design26.
In this article, we identify practical features of AI development, that have crucial importance for translation, and define their vertical integration within an AI ‘supply chain’. We demonstrate how vertically integrated approaches can work in practice, through the lens of two successful, contrasting, real-world pipelines: a major, scalable, AI platform in a high-resource setting (Mayo Clinic, Rochester, United States), and a focused, embedded AI system for cervical cancer screening in a low-resource setting (Paps AI, Mbarara, Uganda). We discuss challenges and provide recommendations that can help future AI projects cross the gap from pages of medical journals to patient bedsides.
Outside of the algorithm
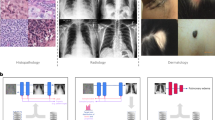
Increasing attention is being paid to translational aspects of clinical AI27. Recent frameworks28 and maturity classifications in literature reviews1,29 adopt a high-level view of where an algorithm sits in its development roadmap. These supplement checklists for risk of bias and reporting that are internal to algorithm training and evaluation, for prediction30 and diagnostic accuracy31, which focus on model-building32 and generalisability33. However, where AI development is intended to lead to clinical deployment, success also depends on practical considerations34 outside of model-building (Fig. 1), including impact evaluation, data lifecycles, and production. In the following sections, we describe the contribution of each stage.
Impact evaluation
AI usage in non-healthcare industries is predicated on creation of ‘value’, measured as tangible return-on-investment. Medical AI is less mature, and most research focuses on accuracy in experimental datasets. Evidence for comparative performance against an existing non-AI gold standard (e.g., AI vs radiologist, or AI + radiologist vs radiologist) is sparser, while evidence of tangible impact on patients or cost-effectiveness is severely lacking. Implementation should aim to generate value for end-users and patients. This could derive from AI-assisted insights with no real-world equivalent (such as associations in complex data), or from augmenting existing skilled clinicians. The latter is particularly relevant for low-income, low-resource environments, or high-resource areas where workstreams are bottlenecked by particular tasks. Impact evaluation therefore includes establishing and estimating clear end-user or patient-centred outcome targets before building a model, as well as planning and monitoring for unintended, post-deployment effects such as over-investigation and over-treatment, or costs from safety-netting high-risk decisions35. Involvement of end-users from implementation environments is vital to evaluate real-world impacts, above and beyond a traditional focus on model accuracy36.
Data lifecycles
A healthcare data lifecycle describes generation, curation and aggregation, and maintenance of patient data that is used by consumers (such as clinicians and researchers) and patients themselves37. Practical examples can be seen in Learning Healthcare Systems, where analysis is built into daily practice38. A lifecycle view emphasises data flows, where data is constantly produced during routine care, and where use and utility of data is often time-constrained. In contrast, model-building is traditionally performed on static datasets that have passed through often unreproducible, proprietary, processing steps. More data, and external data, is not necessarily useful, as additional features may only be available in research settings or through manual collection. While the importance of representative training data is well recognised, other factors can impede successful deployment. These include: (1) differences in how data is acquired and processed, between curated datasets and live implementation environments39 (for example – heterogeneous imaging protocols40, input and coding of electronic data41,42,43, quality of acquisition device21); (2) software and hardware requirements to stream data to a model, which may vary from simple DICOM ingestion to integrating multi-modal data from multiple devices; and (3) a more comprehensive scope of raw data and signals in a live environment, that are not considered by a model, but provide additional insights to a diagnostician (thus reducing relative AI performance).
AI in production
Production describes the process of bringing an AI model to active deployment. Technical requirements in this stage (including backend/frontend development and product delivery, or “DevOps”), usually call on the expertise of deployment engineers and software developers. As a result, once a promising algorithm has been evaluated by researchers, it will require additional expertise to insert it into the midst of competing software and hardware infrastructure. Successful production enables data ingestion by a model, provides computational power, presents an interface to observe the model working, and returns insights to users. In practice, no two production environments look the same. For example, a model can be embedded into a software application with its own codebase, internal data lifecycle, and user interface. Many imaging algorithms deploy into commonly used radiology workflow software (as seen with segmentation algorithms6). Similarly, a model can integrate into physical devices with their own computational power and interface (for example, arrhythmia detecting smartwatches44,45). Embedding models takes advantage of discrete workflows and simpler data lifecycles but are ‘locked-in’ to specific uses. A model can instead be served as a module within a larger system, communicating with other modules via interoperable data formats. This approach is scalable, and maintains control over compute, heterogeneous data input/output, flexibility in technology, and resilience in upgrading. However, this comes with high set-up costs and complex development, typically requiring whole organisational buy-in. For models requiring rich data from multimodal sources, this may be the only viable route. Other production considerations include ability to monitor for software bugs and hardware failures, and observe changes in real-world circumstances and data distributions that may cause model performance to deteriorate (‘model drift’) with potential for harm. Models brought to production can encounter difficulties in data interoperability and hardware compatibility, particularly in complex clinical software environments.
Summary
By the time an AI model, robust to external dataset validation, enters the deployment stage, it may be too late to address challenges related to insufficient clinical impact, inadequate data, and difficulties in production that were not considered in a model-centric approach. These challenges are as important as model-building, with respect to potential for implementation.
Vertically integrating an AI ‘supply chain’
Vertical integration is a concept from industry that has existed since the 19th century46, recognising that supply chain components are co-dependent, and that flow of requirements and information is not unidirectional47. Vertical integration synchronises each stage, lowering transaction time and cost to move between them, and renders the entire chain less vulnerable to failure from not anticipating the needs of any individual stage.
We can conceptualise AI development as a vertically integrated supply chain, where model-building is analogous to product construction and testing (Fig. 1). As with traditional product supply chains, this stage does not exist in a vacuum. Rather, operationalisation is entirely dependent on well-functioning components across the chain. Additionally, components must continue to work synergistically to support a deployed model (for example: on-going evaluation of clinical pathway impact, data lifecycles for observation/re-validation, and production environments responsive to safety issues and end-user feedback).
We therefore summarise vertically integrated AI as a holistic approach to AI development, where a focus across the entire supply chain can lead to ready-to-implement models, that are less vulnerable to component failures. In practice, this calls for three actions:
-
(1)
To work across all supply chain components in parallel from the planning stage, by aligning to requirements of the final product.
-
(2)
To move beyond academically focused groups to cross-disciplinary teams, where end-users, developers and deployment engineers, and implementation experts, play as significant a role as clinician scientists and data scientists.
-
(3)
Developing a strategy within research groups, provider organisations, or technology companies, that can facilitate these processes.
For teams looking to create a clinically deployable model, these translate to key considerations in Fig. 2. In the following sections we discuss how this approach is implemented in two AI pipelines with extreme divergence in setting and use-case.
The Mayo Clinic AI factory – a ‘whole systems’ vertically integrated approach
One strategy for vertical integration is to build an entire organisation-wide infrastructure around AI development. The Mayo Clinic AI factory hosts cross-disciplinary expertise and a development platform, that minimises distance from concept to implementation48. Platform architecture is illustrated in Fig. 3. In summary, an interoperable data middle layer (“Gather”) utilises multiple Fast Healthcare Interoperability Resource (FHIR) application programming interfaces (APIs) to receive EHR and device/wearables data, with additional APIs integrating imaging and signal (e.g. electrocardiogram) data. Data is hosted in the cloud (Google Cloud Services, Mountain View, USA), where reproducible harmonization and quality assurance enables data consistency. Model-builders can access data through the “Discover” component, which provides a development environment with compute infrastructure and software tools. Trained models are passed to “Validate”, which facilitates silent evaluation on prospective data streams, and automates assessment of model bias by evaluating across population subgroups, and in benchmark datasets for sociodemographic characteristics. “Validate” reports performance across a range of scenarios, including calibration and potential for bias in marginalized populations.
This includes components for data curation (“Gather”), data access and analytics (“Discover”), model validation (“Validate”) and an on platform production environment (“Deliver”). This approach, whilst costly, greatly reduces distance from concept to deployment. Cross-disciplinary working is a vital component external to the illustrated architecture.
Integration of software and computational hardware onto a single platform simplifies production, as implementation can be enabled by switching on a “Deliver” component. Model outputs are translated into insights via pre-defined rules, sent to end-users using the existing messaging APIs interfacing with EHR and devices. End-users may be presented with flags, personalized care plans, or access to relevant guidance. Finally, outcome indicators belong to the same data lifecycle and are used to estimate potential impact, or measure intended and unintended post-deployment impacts. The entire platform is supported by a cross-disciplinary team who work with end-users to identify areas of maximal impact, and to vet feasibility with respect to data and production requirements.
A major challenge in implementing a ‘whole systems’ platform is patient privacy. In addition to removing known identifying elements from multimodal data, the platform employs best-on-class de-identification protocols for EHR data (nference, Cambridge, USA)49. A “data behind glass” approach places authorized sub-tenants in encrypted containers (under Mayo control) but does not allow data to leave containers, preventing merging data with external sources for re-identification.
Launch of the platform in 2020 has resulted in a rich development pipeline50. A case-study can be made of ECG-guided screening for asymptomatic left ventricular systolic dysfunction which was taken from conception, through one of the largest randomized control trials of an AI device to date (EAGLE)51, and is now undergoing pilot implementation and validation under Food and Drug Administration breakthrough designation. Development is summarised in Table 1, but in short, key contributors to success include: (1) a cross-disciplinary feasibility and planning process; (2) development and deployment supported by interoperable data flows; (3) existing infrastructure that supports regulatory conformity for the whole product lifecycle.
Other use-cases evaluated on the platform include portable ECG assessment52, prediction of post-surgical mortality53, and real-time monitoring of COVID-19 interventions54. More than 200 additional models are in different stages of development maturity55, while the platform additionally hosts and accelerates start-ups to market readiness56. Vertical integration of data, modelling and validation, production, and clinical impact evaluation into a single platform bridges the gap between algorithms and implementation.
PapsAI - vertical integration in response to resource scarcity
In an opposing, resource-poor scenario, a vertically integrated approach means prioritising infrastructure, and planning for challenging environments where there may not be easy paths to translating model outputs into actions. In such settings, a focus on model-building may produce an algorithm with excellent performance across multiple datasets, but will not address implementation barriers (Fig. 4).
Uganda has high cervical cancer incidence and mortality, with lack of screening resources (included trained cytopathologists) contributing to late diagnosis57. Existing algorithms are trained on datasets for high-resource economies with different demographic and data quality characteristics (including cleaner slide preparation) and are designed to integrate with Western cytopathological workflows and expensive devices58. Despite a clear use-case for AI, development must contend with a lack of infrastructure. The locally developed approach taken by William et al.59 has involved parallel development of hardware and software to support local data lifecycles, with portable training, validation, and production environments, and a health record for outcomes data (Fig. 5), resulting in a ‘ready-to-deploy’ system.
In contrast to Fig. 4, a focus on building supply chain components that support a predictive model will ensure that the model can be operationalized in the real world.
In summary, cytopathological images are acquired through 3D-printed components integrating a microscope slide scanner, networked to an image storage system with labelling/training environment. With parallels to the Mayo Clinic platform (although at a much smaller scale), the same locally applicable data flows are used for model validation. Models are calibrated to local images, include artefact handling, with ability to run on low-end hardware. The production environment sits alongside training software on mobile, low-power devices, and integrates the same data flows. Outputs are added to an electronic record that can be viewed by patients or clinicians and linked to treatment and outcomes. Finally, acquired images can be manually assessed to re-validate the model and enrich the dataset.
There is a paucity of AI research in low to low-middle income countries (LLMIC)1, where there is also significant lack of diagnostic resource. Vertical integration promotes local infrastructure – a pre-requisite for representative data and implementation environments. With care, LLMIC AI development can achieve substantially over par return-on-investment, when compared to sums invested into AI for well-functioning clinical pathways in high-resource areas.
Challenges and solutions in vertical integration
Artificial intelligence has captured the imagination of clinicians and researchers, funders, and commercial investors; but without widespread translation to clinical impact, we risk disillusionment and a collapse in willingness to invest resources.
Vertical integration describes a holistic approach to AI, which engages with all supply components of a planned product at conception, employs teams with cross-disciplinary expertise, and adopts a strategic recognition that model-building in isolation, while often a substantial academic achievement, is not always a practical one.
Practically, approaches will vary across settings. The distance between model-building and other components is a spectrum that differs across data types and clinical environments. The described cases represent two extremes: a large-scale transformation across an organisation, and a planned approach to maximise potential for operationalization in a resource-poor setting. In many other cases, substantial infrastructural changes are unnecessary, as deployment requirements are lower. For example, the dominance of radiomics in development maturity1 and devices8, may reflect lower implementation requirements from standardized data (DICOM) and pre-existing assisted reporting environments. In addition, the components we describe are not in themselves novel. For companies producing AI software-as-medical-devices, a focus on elements such as software-embedding and value demonstration is necessitated by commercial and regulatory drivers. However, a common feature of the clinical AI research translational gap remains separation of dataset experimentation with ability to operationalise models, as seen in lack of candidates for clinical translation amongst hundreds of COVID-19 models with high reported accuracy60,61.
Addressing additional, specific challenges can also help transform current approaches. First, priority for AI funding should be given to proposals with integrated roadmaps to implementation. Statistical methodology could be supplemented by understanding of informatics infrastructure, involvement of deployment experts, and assessment or estimation of longer-term impact. This can be seen in practice, where NHS Transformation in the UK funds projects that fulfil urgent care priorities, and demonstrate feasibility of workflow deployment62.
Second, transition to an entirely vertically integrated platform like Mayo Clinic requires whole organisation buy-in. While this can produce ground-breaking results, the required organisational transformation and investment may be unfeasible. Centralisation may be an alternative in regional healthcare networks, accumulating cross-disciplinary expertise from multiple centres, while creating population-level data flows, often through the use of the commercial platform providers63. A diametrically opposite approach is decentralisation using federated architectures to manage data environments across multiple organisations, each hosting local cross-disciplinary teams, as being developed by the London Medical Imaging & AI Centre for Value Based Healthcare (AI4BH)64,65. While centralisation provides economies of scale for technical specialisation, decentralisation aims to harness synergies through proximity to domain experts and data sources for local requirements.
Finally, medical device regulation and safety monitoring requires reconsideration. Proposed regulation in the USA and UK for a ‘product lifecycle approach’ will consider data flows and production practices (alongside experimental performance metrics)66,67. Vertical integration actively supports meeting these regulatory requirements but may also benefit from guidance to address challenging issues such as model and dataset drift, and on-going quality and risk management systems. This dynamic post-translational management stage has been termed ‘MLOps’ by non-healthcare industries68.
Conclusion
Even externally validated and accurate AI models cannot compensate for practical problems that preclude deployment into real-world workflows. Clinical AI development must vertically integrate cross-disciplinary teams and supply chain components that directly support model implementation. This broad approach is adaptable to different settings and can help improve translation of clinical AI research into clinical workflows.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
References
Zhang, J. et al. An interactive dashboard to track themes, development maturity, and global equity in clinical artificial intelligence research. Lancet Digital Health 4, e212–e213 (2022).
Pretnik, R. & Krotz, L. Healthcare AI 2020. https://klasresearch.com/report/healthcare-ai-2020-investment-continuesbut-results-slower-than-expected-a-decision-insights-report/1443 (2020).
Rob, B. et al. Top of Mind for Top Health Systems. https://paddahealth.com/wpcontent/uploads/2020/11/Top_of_Mind_for_Top_Health_Systems_2021_CCM_Reports_FINAL.pdf (2020).
Balakrishnan, T., Chui, M., Hall, B. & Henke, N. The State of AI in 2020. https://www.mckinsey.com/business-functions/quantumblack/ourinsights/global-survey-the-state-of-ai-in-2020 (2020).
Lavender, J. Venture Pulse: Investment in AI for healthcare soars. https://home.kpmg/xx/en/home/insights/2018/04/venture-pulse-q1-18-globalanalysis-of-venture-funding.html (2018).
Benjamens, S., Dhunnoo, P. & Meskó, B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. npj Digit. Med. 3, 118 (2020).
Wu, E. et al. How medical AI devices are evaluated: Limitations and recommendations from an analysis of FDA approvals. Nat. Med. 27, 582–584 (2021).
Lyell, D., Coiera, E., Chen, J., Shah, P. & Magrabi, F. How machine learning is embedded to support clinician decision making: an analysis of FDA-approved medical devices. BMJ Health Care Inf. 28, e100301 (2021).
McKinney, S. M. et al. International evaluation of an AI system for breast cancer screening. Nature 577, 89–94 (2020).
Freeman, K. et al. Use of artificial intelligence for image analysis in breast cancer screening programmes: Systematic review of test accuracy. BMJ n1872. https://doi.org/10.1136/bmj.n1872 (2021).
Nagendran, M. et al. Artificial intelligence versus clinicians: systematic review of design, reporting standards, and claims of deep learning studies. BMJ m689. https://doi.org/10.1136/bmj.m689 (2020).
Aggarwal, R. et al. Diagnostic accuracy of deep learning in medical imaging: A systematic review and meta-analysis. npj Digit. Med. 4, 65 (2021).
Shen, J. et al. Artificial intelligence versus clinicians in disease diagnosis: Systematic review. JMIR Med. Inf. 7, e10010 (2019).
Andrew Ng. MLOps: From model-centric to data-centric AI. https://www.deeplearning.ai/wpcontent/uploads/2021/06/MLOps-From-Model-centric-to-Data-centric-AI.pdf (2021).
Lowe, D. Machine Learning Deserves Better Than This. https://www.science.org/content/blog-post/machine-learning-deserves-better (2021).
Navarro, C. L. A. et al. Risk of bias in studies on prediction models developed using supervised machine learning techniques: Systematic review. BMJ 375, n2281 (2021).
Wilkinson, J. et al. Time to reality check the promises of machine learning-powered precision medicine. Lancet Digital Health 2, e677–e680 (2020).
Panch, T., Mattie, H. & Celi, L. A. The “inconvenient truth” about AI in healthcare. npj Digit. Med. 2, 77 (2019).
Wawira Gichoya, J., McCoy, L. G., Celi, L. A. & Ghassemi, M. Equity in essence: a call for operationalising fairness in machine learning for healthcare. BMJ Health Care Inf. 28, e100289 (2021).
MI in Healthcare Workshop Working Group. Machine intelligence in healthcare—perspectives on trustworthiness, explainability, usability, and transparency. npj Digit. Med. 3, 47 (2020).
Beede, E. et al. A Human-Centered Evaluation of a Deep Learning System Deployed in Clinics for the Detection of Diabetic Retinopathy. in Proceedings of the 2020 CHI Conference on Human Factors in Computing Systems 1–12 (ACM,). https://doi.org/10.1145/3313831.3376718 (2020).
Strickland, E. IBM Watson, heal thyself: How IBM overpromised and underdelivered on AI health care. IEEE Spectr. 56, 24–31 (2019).
Wong, A. et al. External Validation of a Widely Implemented Proprietary Sepsis Prediction Model in Hospitalized Patients. JAMA Intern. Med. 181, 1065 (2021).
Cam, A. Chui, M. & Hall, B. Global AI Survey: AI proves its worth, but few scale impact. (2019).
Rao, A. & Verweij, G. Global Artificial Intelligence Study: Exploiting the AI Revolution.
Dang, Y., Lin, Q. & Huang, P. AIOps: Real-World Challenges and Research Innovations. In 2019 IEEE/ACM 41st International Conference on Software Engineering: Companion Proceedings (ICSE-Companion) 4–5 (IEEE, 2019). https://doi.org/10.1109/ICSE-Companion.2019.00023.
Kelly, C. J., Karthikesalingam, A., Suleyman, M., Corrado, G. & King, D. Key challenges for delivering clinical impact with artificial intelligence. BMC Med. 17, 195 (2019).
Reddy, S. et al. Evaluation framework to guide implementation of AI systems into healthcare settings. BMJ Health Care Inf. 28, e100444 (2021).
Gallifant, J. et al. Artificial intelligence for mechanical ventilation: systematic review of design, reporting standards, and bias. British Journal of Anaesthesia S0007091221006206, https://doi.org/10.1016/j.bja.2021.09.025 (2021).
Collins, G. S. et al. Protocol for development of a reporting guideline (TRIPOD-AI) and risk of bias tool (PROBAST-AI) for diagnostic and prognostic prediction model studies based on artificial intelligence. BMJ Open 11, e048008 (2021).
Sounderajah, V. et al. Developing a reporting guideline for artificial intelligence-centred diagnostic test accuracy studies: the STARD-AI protocol. BMJ Open 11, e047709 (2021).
Berisha, V. et al. Digital medicine and the curse of dimensionality. npj Digit. Med. 4, 153 (2021).
Futoma, J., Simons, M., Panch, T., Doshi-Velez, F. & Celi, L. A. The myth of generalisability in clinical research and machine learning in health care. Lancet Digital Health 2, e489–e492 (2020).
Arnold, M. et al. Towards Automating the AI Operations Lifecycle. arXiv:2003.12808 [cs] (2020).
Adamson, A. S. & Welch, H. G. Machine learning and the cancer-diagnosis problem — No gold standard. N. Engl. J. Med. 381, 2285–2287 (2019).
Wolff, J., Pauling, J., Keck, A. & Baumbach, J. Systematic Review of Economic Impact Studies of Artificial Intelligence in Health Care. J. Med. Internet Res. 22, e16866 (2020).
Zhang, J. et al. Best practices in the real-world data life cycle. PLOS Digit. Health 1, e0000003 (2022).
Budrionis, A. & Bellika, J. G. The learning healthcare system: Where are we now? A systematic review. J. Biomed. Inform. 64, 87–92 (2016).
Whebell, S. & Zhang, J. Bringing biological ARDS phenotypes to the bedside with machine-learning-based classifiers. Lancet Respiratory Med. 10, 319–320 (2022).
Mali, S. A. et al. Making radiomics more reproducible across scanner and imaging protocol variations: A review of harmonization. Methods JPM 11, 842 (2021).
Huser, V., Williams, N. D. & Mayer, C. S. Linking provider specialty and outpatient diagnoses in medicare claims data: Data quality implications. Appl Clin. Inf. 12, 729–736 (2021).
Dakka, M. A. et al. Automated detection of poor-quality data: case studies in healthcare. Sci. Rep. 11, 18005 (2021).
Sholle, E. T. et al. Underserved populations with missing race ethnicity data differ significantly from those with structured race/ethnicity documentation. J. Am. Med. Inform. Assoc. 26, 722–729 (2019).
Maille, B. et al. Smartwatch electrocardiogram and artificial intelligence for assessing cardiac-rhythm safety of drug therapy in the COVID-19 pandemic. The QT-logs study. Int. J. Cardiol. 331, 333–339 (2021).
Kwon, J. et al. Artificial Intelligence-Enhanced Smartwatch ECG for Heart Failure-Reduced Ejection Fraction Detection by Generating 12-Lead ECG. Diagnostics 12, 654 (2022).
Brown, M. & McCool, B. P. Vertical integration: exploration of a popular strategic concept. Health Care Manag. Rev. 11, 7–19 (1986).
Kumpe, T. & Bolwijn, P. T. Manufacturing: The New Case for Vertical Integration. 8, 75 (1988).
Mayo Clinic Platform. Mayo Clinic Platform: Products and Services. Mayo Clinic Platform https://www.mayoclinicplatform.org/products-and-services/ (2021).
Murugadoss, K. et al. Building a best-in-class automated de-identification tool for electronic health records through ensemble learning. Patterns 100255. https://doi.org/10.1016/j.patter.2021.100255 (2021).
Hannah Mitchell. Mayo Clinic AI factory has dozens of projects underway. Becker’s Hospital Review https://www.beckershospitalreview.com/innovation/mayo-clinic-ai-factory-has-dozens-of-projects-underway.html (2021).
Yao, X. et al. Artificial intelligence–enabled electrocardiograms for identification of patients with low ejection fraction: a pragmatic, randomized clinical trial. Nat. Med. 27, 815–819 (2021).
Giudicessi, J. R. et al. Artificial Intelligence–Enabled Assessment of the Heart Rate Corrected QT Interval Using a Mobile Electrocardiogram Device. Circulation 143, 1274–1286 (2021).
Mahayni, A. A. et al. Electrocardiography-Based Artificial Intelligence Algorithm Aids in Prediction of Long-term Mortality After Cardiac Surgery. Mayo Clin. Proc. 96, 3062–3070 (2021).
McMurry, R. et al. Real-time analysis of a mass vaccination effort confirms the safety of FDA-authorized mRNA vaccines for COVID-19 from Moderna and Pfizer/BioNtech. http://medrxiv.org/lookup/doi/10.1101/2021.02.20.21252134 (2021).
Mayo Clinic. Mayo Clinic: Emerging Capabilities in the Science of Artificial Intelligence. Mayoclinic.org https://www.mayoclinic.org/giving-to-mayo-clinic/our-priorities/artificial-intelligence (2021).
Susan Barber Lindquist. Mayo Clinic Platform_Accelerate program begins with four AI startups. Mayo Clinic News Network https://newsnetwork.mayoclinic.org/discussion/3-23-mayo-clinic-platform_accelerate-program-begins-with-four-ai-startups/ (2022).
Nakisige, C., Schwartz, M. & Ndira, A. O. Cervical cancer screening and treatment in Uganda. Gynecologic Oncol. Rep. 20, 37–40 (2017).
William, W., Ware, A., Basaza-Ejiri, A. H. & Obungoloch, J. A review of image analysis and machine learning techniques for automated cervical cancer screening from pap-smear images. Computer Methods Prog. Biomedicine 164, 15–22 (2018).
William, W., Ware, A., Basaza-Ejiri, A. H. & Obungoloch, J. A pap-smear analysis tool (PAT) for detection of cervical cancer from pap-smear images. BioMed. Eng. OnLine 18, 16 (2019).
AIX-COVNET et al. Common pitfalls and recommendations for using machine learning to detect and prognosticate for COVID-19 using chest radiographs and CT scans. Nat. Mach. Intell. 3, 199–217 (2021).
Wynants, L. et al. Prediction models for diagnosis and prognosis of covid-19: systematic review and critical appraisal. BMJ m1328. https://doi.org/10.1136/bmj.m1328 (2020).
Dan Bamford & Samantha Gan. NHS X - AI in Health and Care Award. (2020).
Richard Torbett. Models of Access to Health Data in the UK. (2022).
Rieke, N. et al. The future of digital health with federated learning. npj Digit. Med. 3, 119 (2020).
AI Centre for Value Based Healthcare. AI4VBH: Platforms. https://www.aicentre.co.uk/platforms#view2 (2022).
US FDA Center for Devices and Radiological Health. Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD) Action Plan. (2021).
Medicines & Healthcare Products Regulatory Agency. Software and AI as a Medical Device Change Programme. (2021).
John, M. M., Olsson, H. H. & Bosch, J. Towards MLOps: A Framework and Maturity Model. in 2021 47th Euromicro Conference on Software Engineering and Advanced Applications (SEAA) 1–8 (IEEE, 2021). https://doi.org/10.1109/SEAA53835.2021.00050.
Acknowledgements
J.Z. acknowledges funding from the Wellcome Trust (203928/Z/16/Z) and support from the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College NHS Trust and Imperial College London. S.B. acknowledges funding from the Wellcome Trust (566701).
Author information
Authors and Affiliations
Contributions
Conception: J.Z., S.B., W.W., J.H., J.T.T.; Methodology: J.Z., S.B., W.W., P.C., H.Shuaib, H.Sood, H.A., J.H., J.T.T.; First draft: J.Z., S.B., W.W., P.C., H.S., J.H.; Subsequent and final draft: J.Z., S.B., W.W., P.C., H.Shuaib, H.Sood, H.A., J.H., J.T.T. All authors are accountable for all aspects of this work.
Corresponding author
Ethics declarations
Competing interests
H.A. declares no Competing non-financial interests, but the following Competing financial interests. H.A. is employed as Chief Scientific Officer, Preemptive Medicine and Health Security, Flagship Pioneering. All other authors declare that they have no non-financial or financial Competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, J., Budhdeo, S., William, W. et al. Moving towards vertically integrated artificial intelligence development. npj Digit. Med. 5, 143 (2022). https://doi.org/10.1038/s41746-022-00690-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41746-022-00690-x
This article is cited by
-
Potential of Artificial Intelligence to Accelerate Drug Development for Rare Diseases
Pharmaceutical Medicine (2024)
-
On the Analyses of Medical Images Using Traditional Machine Learning Techniques and Convolutional Neural Networks
Archives of Computational Methods in Engineering (2023)