Abstract
Artificial intelligence (AI) has been successfully exploited in diagnosing many mental disorders. Numerous systematic reviews summarize the evidence on the accuracy of AI models in diagnosing different mental disorders. This umbrella review aims to synthesize results of previous systematic reviews on the performance of AI models in diagnosing mental disorders. To identify relevant systematic reviews, we searched 11 electronic databases, checked the reference list of the included reviews, and checked the reviews that cited the included reviews. Two reviewers independently selected the relevant reviews, extracted the data from them, and appraised their quality. We synthesized the extracted data using the narrative approach. We included 15 systematic reviews of 852 citations identified. The included reviews assessed the performance of AI models in diagnosing Alzheimer’s disease (n = 7), mild cognitive impairment (n = 6), schizophrenia (n = 3), bipolar disease (n = 2), autism spectrum disorder (n = 1), obsessive-compulsive disorder (n = 1), post-traumatic stress disorder (n = 1), and psychotic disorders (n = 1). The performance of the AI models in diagnosing these mental disorders ranged between 21% and 100%. AI technologies offer great promise in diagnosing mental health disorders. The reported performance metrics paint a vivid picture of a bright future for AI in this field. Healthcare professionals in the field should cautiously and consciously begin to explore the opportunities of AI-based tools for their daily routine. It would also be encouraging to see a greater number of meta-analyses and further systematic reviews on performance of AI models in diagnosing other common mental disorders such as depression and anxiety.
Similar content being viewed by others
Introduction
Mental disorders affect a person’s psychological, social, behavioral, and emotional wellbeing1. The impact of mental disorders is not exclusive to the mind; one’s mental health state affects physical wellbeing and vice-versa2. Globally, mental disorders account for 7% of all total disability-adjusted life years (DALYs) and affect more than 1 billion people, especially those living in high and upper-middle-income nations3. This burden is further exacerbated by the fact that up to 50% and 90% of people with mental disorders receive no treatment in high-income countries and low resource settings, respectively4.
Diagnosing mental disorders is complicated by heterogeneity in clinical presentation, symptomatology, and fluctuations in the course of illness, further compounded by gaps in our understanding of etiological mechanisms. Current practices to diagnose mental disorders rely on frameworks outlined in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) and the International Classification of Diseases (ICD-11) manual. Diagnosis is based entirely on subjective accounts from patients on the one hand and observations and interpretations made by clinicians on the other; objective measures are still not available5. Furthermore, diagnosing mental disorders can be time- and resource-intensive via administering diagnostic tools, conducting interviews with relatives or caregivers, and taking health histories.
Digital health tools and technologies offer great opportunities to support and augment diagnostic and interventional aspects of psychiatric care6. A leading and popular form of such digital technologies is artificial intelligence (AI), which enables machines to learn complex, latent rules and provide actionable conclusions through understanding queries and sifting through and connecting mountains of data points7. Advances in the use of AI for diagnostic and therapeutic mental health interventions are on the rise with multiple examples including social bots to support dementia care, sexual disorders, and even virtual psychotherapists8,9,10,11. AI has great potential to reshape our understanding of mental disorders and how to diagnose them. Leveraging AI to study and make sense of complex patterns and interactions between one’s genes, brain, behaviors, and experiences present an unprecedented opportunity to improve early mental illness detection and personalize treatment options5.
There have been a wealth of studies examining the accuracy of AI models in diagnosing mental disorders such as Alzheimer’s Disease (AD)12, schizophrenia (SCZ)13, bipolar disorders (BD)14, posttraumatic stress disorders (PTSD)15, and obsessive-compulsive disorder (OCD)16. Numerous systematic reviews summarize the evidence resulting from these studies. Although conducting an umbrella review (i.e., a review of systematic reviews) is important to draw more accurate and comprehensive conclusions on a particular topic, to our knowledge, no previous umbrella reviews were published to summarize the evidence about diagnostic performance of AI models for mental disorders. This umbrella review aims to synthesize the previously published evidence on the performance of AI models in diagnosing mental disorders.
Results
Search Results
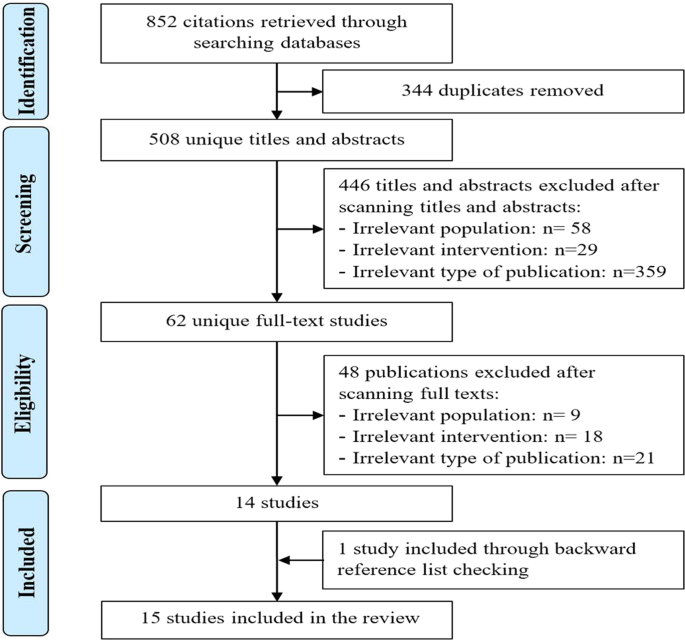
As presented in Fig. 1, we identified a total of 852 citations from searching the bibliographic databases. The software EndNote identified and removed 344 duplicates of the retrieved citations. Screening titles and abstracts of the remaining 508 citations led to excluding 446 citations. By reading the full text of the remaining 62 publications, we excluded 48 publications. An additional systematic review was identified through checking the list of the included reviews. In total, 15 systematic reviews were included in the current review17,18,19,20,21,22,23,24,25,26,27,28,29,30,31.
Of these, 344 duplicates were removed. Screening titles and abstracts of the remaining citations led to excluding 446 citations. By reading the full text of the remaining 62 publications, we excluded 48 publications. An additional systematic review was identified by checking the list of the included reviews. In total, 15 systematic reviews were included in the current.
Characteristics of included reviews
Interestingly, the included reviews were published between 2017 and 2020, and more than half of them (n = 8) were published in 2020 (Table 1). The included reviews were conducted in 7 different countries, but more than half of them were conducted in Italy (n = 5) and the United Kingdom (n = 4). All included reviews were articles in peer-reviewed journals. Only four reviews had a registered protocol. All studies except one stated that they followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
With regards to the eligibility criteria, the included studies focused on diagnosing 10 mental disorders, namely: Alzheimer’s disease (AD) (n = 7), mild cognitive impairment (MCI) (n = 6), and Schizophrenia (SCZ) (n = 3) (Table 2). While seven reviews focused on any AI approach, another seven reviews focused merely on supervised machine learning (SML), and one review focused on deep learning (DL). SML uses labeled datasets to train algorithms in order to predict or label new, unforeseen examples, SML is used for classification and regression purposes. UML analyzes unlabeled data to discover hidden features, patterns, and relationships in data. Clustering, association, and dimensionality reduction are three major applications of unsupervised learning models. It is worth mentioning that most deep learning applications are based on supervised learning. More than half of the reviews (n = 8) focused on neuroimaging data for diagnosing mental disorders. While seven reviews restricted the search to studies in the English language, there was no language restriction imposed in six studies. Eight studies applied time restrictions to the search while the remaining studies did not.
Varied numbers of electronic databases were searched in the included reviews. The most common databases used in the included reviews are MEDLINE (n = 13), Web of Science (n = 7), EMBASE (n = 6), PsycINFO (n = 5), and Scopus (n = 4) (Table 3). Eight studies used either backward reference list checking (n = 7) or forward reference list checking (n = 1) to identify further studies. Two independent reviewers carried out the study selection process in twelve reviews, performed data extraction in four reviews, and assessed study quality in two reviews. The quality of studies was assessed in nine reviews using six different tools such as a revised tool for Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) and Jadad rating system. Four reviews synthesized the data using meta-analysis.
The number of retrieved studies in the included reviews ranged from 52 to 7,991 (Table 4). The number of included studies in the included reviews varied between twelve to 114. The size of data sets used to train and validate models in the included studies ranged between 10 and 7,026 data points. The included studies in the included reviews used different types of data to train and validate models, namely: neuroimaging data (n = 13), neuropsychological data (n = 6), genetic data (n = 4), and Electroencephalography (EEG) measures (n = 4). As shown in Table 5, many methods were used in the included studies, and the most common ones were Support Vector Machine (SVM) (n = 13), Random Forest (RF) (n = 10), Naïve Bayes (NB) (n = 7), k-Nearest Neighbors (k-NN) (n = 5), and Linear Discriminant Analysis (LDA) (n = 5). The models in the included reviews were validated using only internal validation methods (n = 6) or both internal and external validation methods (n = 3).
Results of study quality appraisal
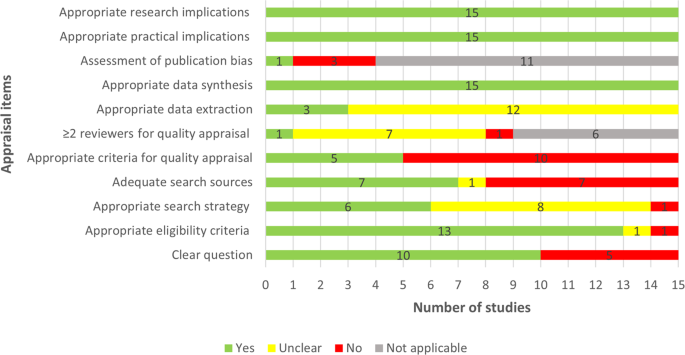
Two thirds of the included reviews clearly stated the review question or aim by identifying the AI approach of interest and its aim, the target disease, and type of data for the model development (Fig. 2). The eligibility criteria were detailed, clear, and matched the review question in 13 reviews. Six studies showed a clear and adequate search strategy that contained all search terms related to the topic, Subject Headings, and limits. Less than half (n = 7) of the included reviews used adequate search sources such as searching multiple major databases and backward and forward reference list checking. Only five reviews assessed the quality of the included studies using a tool suitable for the review question. The quality assessment was carried out by two or more reviewers independently in only a single review. In three reviews, bias and errors in data extraction were minimal, given that at least two reviewers independently extracted the data using a piloted tool. Publication bias and its potential impact on the findings were assessed in only one review. All included reviews used an adequate approach for data synthesis and provided relevant research and practical implications based on the findings. Supplementary Table 1 shows reviewers’ judgments about each appraisal item for each included review.
Yes (green) refers that study meets the item, thereby, it has a good quality in terms of that item. No (red) refers that study did not meet the item, thereby, it has poor quality in terms of that item. Unclear (yellow) refers that we could not appraise the study in terms of the item due to the lack of reported information. Not applicable (gray) refers that the appraisal item is not applicable to the systematic review as it does not include a feature that the item assesses.
Results of studies
The included reviews assessed the performance of AI models in diagnosing 8 mental disorders: Alzheimer’s disease, mild cognitive impairment, schizophrenia, autism spectrum disorder, bipolar disease, obsessive-compulsive disorder, post-traumatic stress disorder, and psychotic disorders. The performance of the AI models in diagnosing these mental disorders is presented in the next subsections.
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by an ongoing decline in brain functions such as memory, executive functions, and language processing32. Four reviews assessed the performance of AI classifiers in differentiating AD from healthy control (HC) using neuroimaging data17,18,19,20 (Table 6). The number of mutual studies was five between Pellegrini et al.17 and Ebrahimighahnavieh et al.20 and four between Pellegrini et al.17 and Sarica et al.19. Accuracy, sensitivity, and specificity of the classifiers in these four reviews ranged from 56% to 100%, 37.3% to 100%, and 55% to 100%, respectively (Table 6). None of these reviews pooled the results using meta-analysis due to the high heterogeneity in the used classifiers, data types, data features, and types of validation.
Two other reviews examined the performance of AI classifiers in differentiating AD from HC using neuropsychological data21,22. There are four mutual studies between the two reviews. Accuracy of the classifiers in these reviews ranged from 68% to 100% (Table 6). One of these reviews meta-analyzed sensitivities and specificities reported in eleven studies and showed a pooled sensitivity of 92% and a pooled specificity of 86%22.
Three reviews examined the performance of AI classifiers in differentiating AD from mild cognitive impairment (MCI) using neuroimaging data17,18,20 (Table 7). There are five mutual studies between Pellegrini et al.17 and Ebrahimighahnavieh et al.20. Accuracy, sensitivity, and specificity of the classifiers in these three reviews ranged from 56% to 100%, 40.3% to 100%, and 67% to 100%, respectively (Table 7). None of these reviews pooled the results using meta-analysis due to the high heterogeneity. One other review examined the performance of AI classifiers in differentiating AD from MCI using neuropsychological data21. Accuracy of the classifiers in that review varied between 68% to 86% (Table 7).
One review assessed the performance of AI classifiers in differentiating AD from Lewy body dementia (LBD) using EEG measures23. Accuracy, sensitivity, specificity, and AUC of the classifiers in this review ranged from 66% to 100%, 76% to 100%, 77% to 100%, and 78% to 93%, respectively.
Mild cognitive impairment (MCI) refers to deterioration in cognitive functions (e.g., memory, thinking, and language) that is detectable but it is less severe than the deterioration in patients with AD33. MCI represents a transitional stage between the expected cognitive decline associated with normal aging and the more severe decline of dementia33. Four reviews assessed the performance of AI classifiers in differentiating MCI from HC using neuroimaging data17,18,19,20 (Table 8). The number of mutual studies was five between Pellegrini et al.17 and Ebrahimighahnavieh et al.20 and four between Pellegrini et al.17 and Sarica et al.19. Accuracy, sensitivity, and specificity of the classifiers in these four reviews ranged from 47% to 99.2%, 24.3% to 98.3%, and 47.1% to 97%, respectively (Table 8). None of these reviews pooled the results using meta-analysis due to the high heterogeneity.
Two other reviews examined the performance of AI classifiers in differentiating MCI from HC using neuropsychological data21,22. Four studies were mutual studies between the two reviews. Accuracy of the classifiers in these reviews ranged from 60% to 98% (Table 8). Only one of these reviews meta-analyzed sensitivities and specificities reported in nine studies and showed pooled sensitivity and specificity of 83% each22.
Three reviews examined the performance of AI classifiers in differentiating MCI converting to AD (MCIc) from MCI non-converting to AD (MCInc) using neuroimaging data17,19,20 (Table 9). The number of mutual studies was five between Pellegrini et al.17 and Ebrahimighahnavieh et al20 and four between Pellegrini et al.17 and Sarica et al.19. Accuracy, sensitivity, and specificity of the classifiers in these three reviews ranged from 47% to 96.2%, 42.1% to 99%, and 51.2% to 95.2%, respectively (Table 10). None of these reviews pooled the results using meta-analysis due to the high heterogeneity.
Another review examined the performance of AI classifiers in differentiating MCIc from MCInc using neuropsychological data22. Accuracy, sensitivity, specificity, and AUC of the classifiers in this review ranged from 61% to 85%, 50% to 91%, 48% to 91%, and 67% to 93%, respectively. This review meta-analyzed sensitivities and specificities reported in ten studies and showed a pooled sensitivity of 73% and a pooled specificity of 69%.
Schizophrenia (SCZ) is a long-term serious mental disorder, in which patients are not able to differentiate between their thoughts from reality due to disturbances in cognition, emotional responsiveness, and behavior34. Two reviews investigated the performance of AI classifiers in differentiating SCZ from HC using neuroimaging data24,25. There are 15 mutual studies between the two reviews. Accuracy, sensitivity, and specificity of the classifiers in the two reviews ranged from 61% to 99.3%, 57.9% to 100%, and 40.9% to 98.6%, respectively (Table 10). None of these reviews pooled the results using meta-analysis. One review examined the performance of AI classifiers in differentiating SCZ from HC using genetic data26. Accuracy and AUC of the classifiers in this review ranged from 40% to 86% and 54% to 95%, respectively.
Bipolar disorder is a mood disorder that is characterized by mood fluctuations between symptoms of mania or hypomania and depression35. One review assessed the performance of AI classifiers in differentiating bipolar BD from HC using neuroimaging data27. Accuracy, sensitivity, and specificity of the classifiers ranged from 55% to 100%, 40% to 100%, and 49% to 100%, respectively (Table 11). This review examined the performance of AI classifiers in differentiating BD from HC using neuropsychological data27. Accuracy of classifiers varied between 71% and 96.4% (Table 11). This review also investigated the performance of AI classifiers in differentiating BD from major depressive disorder using neuroimaging data. Accuracy, sensitivity, and specificity of the classifiers ranged from 54.76% to 92.1% (n = 7), 57.9 to 83% (n = 3), and 52.1 to 90.9% (n = 3), respectively. Another review used genetic data and AI classifiers to differentiate BD from HC26. Accuracy and AUC of the classifiers ranged from 54% to 77% and 48% to 65%, respectively (Table 11).
Autism spectrum disorder (ASD) is a group of disorders (e.g., autism, childhood disintegrative disorder, and Asperger’s disorder) that starts usually in the preschool period and is characterized by difficulties or impairment in communication and social interaction36. One review investigated the performance of AI classifiers in differentiating ASD from HC using neuroimaging data28. Accuracy, sensitivity, and specificity of the classifiers in the review ranged from 45% to 97%, 24% to 100%, and 21% to 100%, respectively (Table 12). The review meta-analyzed sensitivities and specificities of AI classifiers based on structured MRI (sMRI) in 11 studies. The review found a pooled sensitivity of 83%, a pooled specificity of 84%, a pooled AUC of 90%28. The review also meta-analyzed sensitivities and specificities of deep neural network-based classifiers in one study (five samples) that used functional MRI (fMRI) as a predictor. The review found a pooled sensitivity of 69%, a pooled specificity of 66%, and a pooled AUC of 71%28.
The review assessed the performance of AI classifiers in differentiating ASD from HC using a neuropsychological test (behavior traits)28. Accuracy, sensitivity, and specificity of the classifiers in the review ranged from 78.1% to 100%, 64% to 100%, and 48% to 97%, respectively (Table 12). Further, the review tested the performance of AI classifiers in differentiating ASD from HC using biochemical features28. Accuracy, sensitivity, and specificity of the classifiers in the review ranged from 75% to 94%, 77% to 94%, and 67% to 93%, respectively (Table 12). The review also examined the performance of AI classifiers in differentiating ASD from HC using EEG measures28. Accuracy, sensitivity, and specificity of the classifiers in the review ranged from 85% to 100%, 94% to 97%, and 81% to 94%, respectively (Table 12). The review did not conduct a meta-analysis for the above-mentioned results due to heterogeneity between samples28.
Posttraumatic stress disorder (PTSD) refers to feelings of fear, anxiety, irritability, terror, or guilty that result from remembering very stressful, life-threatening, frightening, distressing events that a patient lived through or witnessed in the past37. One review examined the performance of AI classifiers in differentiating PTSD from HC29. Accuracy of the classifiers using neuroimaging data varied between 89.2% and 92.3% (n = 3). The review also assessed the performance of AI classifiers in differentiating PTSD from trauma-exposed controls29. Accuracy of the classifiers using neuroimaging data varied between 67% and 83.6% (n = 4). Meta-analysis was not carried out in the review.
Obsessive-compulsive disorder (OCD) is a mental health condition in which an individual has frequent intrusive thoughts that lead him or her to perform repetitive behaviors, which may affect daily activities and cause severe distress38. One review assessed the performance of supervised machine learning classifiers in distinguishing OCD from HC using neuroimaging data30. Accuracy, sensitivity, and specificity of the classifiers in the review ranged from 66% to 100% (n = 11), 74.1% to 96.2% (n = 6), and 72.7% to 95% (n = 6), respectively. The review did not pool the results using meta-analysis.
Psychotic disorders are a group of mental disorders in which a patient has incorrect perceptions, thoughts, and inferences about external reality although there is contrary evidence39. One review examined the performance of AI classifiers in differentiating patients with a high risk of developing psychotic disorders from HC using neuroimaging data or neuropsychological tests31. Sensitivity and specificity of the classifiers in the review ranged from 60% to 96% (n = 12) and 47% to 94 (n = 12), respectively. The review meta-analyzed sensitivities and specificities of AI classifiers in 12 studies and found a pooled sensitivity of 78% and a pooled specificity of 77%31.
Discussion
This umbrella review provides an evidence map of the state of the art of AI technologies in diagnosing mental health disorders. The 15 included systematic reviews focused on diagnosing 8 mental disorders. Considering the probability for MCI to progress into clinically diagnosed AD paired with our still limited understanding of contributing factors, it is hardly surprising that more than 200 original studies and 40% of the included reviews focused on AD and MCI.
We also observe that the reported pooled sensitivity of 92% and specificity of 86% for classifying AD vs. HC is higher than for classifying MCI vs. HC (83% pooled sensitivity and specificity), and both are higher than for classifying MCIc vs. MCInc (73% pooled sensitivity and 69% specificity)22. This may be attributed to the fact that AD is a neurodegenerative disease, thereby, there is a continuum ranging from AD on one extreme to HC on the other. Accordingly, discerning extremal cases seems intuitively easier than between more similar stages. This is in line with the reported performances for differentiating PTSD from HC being higher than from trauma-exposed controls29. However, we would also like to point out that the same review reports methods with better performance than the pooled sensitivities and specificities quoted above. This raises the question if such pooling is meaningful from the point of a user, since it obfuscates the existence of better diagnostic tools in the same review.
For classifying SCZ vs. HC, we observe that neuroimaging data tends to lead to better-performing classifiers than genetic data. Unsurprisingly, using genetic data alone leads to significantly lower performance, reflecting that both genetic and environmental factors causing SCZ are described in the literature40. Likewise, classifying BD from HC using genetic data alone shows lower performance. It is interesting to note that for BD vs. HC, neuropsychological data seems to achieve decent accuracy (71%-96.4%) more reliably than neuroimaging data (55%-100%). However, this may also be a result of low sample count (n = 3 using neuropsychological data, n = 8 using neuroimaging data).
For discriminating ASD from HC, most data types can support methods with good accuracy but using biochemical features or EEG measures lead to a significantly increased sensitivity and specificity. Structured MRI leads to better-pooled specificities and sensitivities when compared to functional MRI. This can be attributed to two reasons: (1) sMRI findings resulted from pooling 12 samples from 10 different studies while fMRI resulted from five samples from only two studies, and (2) the deep neural network (DNN) was used as a classifier in the fMRI studies whereas it was used as a classifier in only one sMRI study28.
One review showed promising results regarding the performance of AI models in distinguishing OCD from HC using neuroimaging data. These results should be interpreted carefully for three reasons. First, these results are based on studies with small samples (i.e., 20-172). Second, most included studies used cross validation methods to assess the performance of their models, which is not the most suitable method when the sample size is small. Third, large heterogeneity in OCD patients and the classification features in the included studies.
We found acceptable pooled sensitivity (78%) and pooled specificity (77%) for differentiating patients with a high risk of developing psychotic disorders from HC. However, the authors of that review could not draw a definitive conclusion about applicability of AI models due to high clinical and methodological heterogeneity in meta-analyzed studies.
Reporting practices in the original literature continue to severely hinder statistical meta-analysis of results. On the one hand, the reported up-to-perfect performance for many tasks by the included studies signals a new age of AI, where, given the right modality and amount of data impressive results are reported tasks with real-world significance. However, considering that many original studies seemingly choose performance metrics at random could suggest a definition of success by choice of metric rather than by the task at hand. This, in turn, leaves us with an ambivalent feeling regarding the usefulness of attempts of such analyses (as, e.g., performed by Battista et al.22). Between two competing methods that (a) are properly validated with a large enough cohort, (b) have shown sufficient generalization (e.g., in the form of an external validation) and that (c) use the same data modality, the one with the better performance should be chosen. This underscores the importance of following proper reporting practices, since statistical evaluation (from a clinical, not technological point of view) otherwise seems moot.
The included reviews focused on the performance of AI models in diagnosing 8 mental disorders. However, our search process did not pick up on systematic reviews for several other mental disorders, such as major depressive disorder (MDD), anxiety, eating disorders, and personality disorders. Thus, there is a need to conduct systematic reviews to synthesize the evidence on performance of AI models in diagnosing such mental disorders.
The systematic review of AI studies differentiating high-risk psychosis cases with healthy controls31 is a case example of where the field could benefit from more research. The benefits of early diagnosis could offer the opportunity for intervention prior to full development of a psychotic disorder. Further studies could focus on at-risk groups or identifying ‘at-risk’ for other disorders such as anxiety and MDD and possibly broaden data source types to those that are more accessible and practical than neuroimaging data.
Neuroimaging data for AI models seemed to dominate in the systematic reviews included in this review. In spite of the promising performance of these AI models, we question the practicality of incorporating neuroimaging data into routine diagnostic practice due to it being a resource-intensive procedure. By contrast, AI models of neuropsychological, genetic, and EEG tests could offer exciting opportunities to complement and improve existing diagnostic processes in mental healthcare.
According to the performance reported in the included studies, AI shows a great potential to lead to accelerated, accurate, and more objective diagnoses. The findings in this review strongly suggest that AI is on the jump into clinical use. We believe it is therefore important to educate practitioners exploring the potential for new diagnostic and therapeutic methods as they shift their focus as in so many other jobs that now begin utilizing AI8; this exploratory use should be ethical and cautious. The availability of high-quality AI solutions may even pave the way for an entirely new medical specialization. More important for reliable AI-based classifiers than sample sizes, however, are reproducibility and generality. For a method to be reproducible, data and code must be made available, such that other research teams can verify the code and ensure that the method is free from oversights. For a method to be general, it must deliver results similar to the reported ones on new, previously unseen data. Currently, single site cross-validation is the most common approach; however, validation of new models would benefit greatly from replication using data from external samples.
Many original studies focus on the technical/algorithmic aspects rather than the choice of data modality. This is a consequence of the fact that (supervised) AI is extremely data-hungry, yet high-quality, labeled data is a scarce and expensive resource. It represents a significant amount of effort and manpower. This dependence of contemporary AI on humans dedicating time to first gather and clean, then feed it with data has been likened to a parasitic relationship41,42. As the AI grows, it promises higher utility to humans, which are thus motivated to sift through more data. The temptation to achieve results with the data at hand instead of a thorough investigation into which modality offers the best results is understandably high.
The main limitation of this review is that the data was not synthesized statistically. We could not synthesize the data statistically for three reasons. Firstly, the included reviews were inconsistent in reporting the results of classifier performance. Secondly, most reviews did not extract or present data that is necessary for assessing classifier performance and aggregating the data statistically (i.e., true positive, false positive, true negative, and false negative). Lastly and most importantly, there was high heterogeneity in the AI classifiers (e.g., SVM, DT, RF, CNN, K-NN), data types (e.g., neuroimaging data, genetic data, demographic data), data features (e.g., axial diffusivity, radial diffusivity, mean diffusivity, fractional anisotropy), target mental disorder, model validation approach, and measures of classifier performance reported in the included reviews.
We also do not present the range of performance metrics for classification tasks that were reported by less than three studies. For example, we do not report the classifier performance of AI approaches in distinguishing anorexia nervosa from healthy controls as it was assessed by only one study in one of the included reviews26. Another limitation of this review is that we did not exclude the mutual primary studies between reviews. Therefore, there may be some duplicates in the ranges of classifier performance reported in our review. However, we declared the number of mutual studies between reviews when we aggregated ranges from more than two reviews. We did not exclude reviews based on their quality because most included reviews were judged as low quality in at least four appraisal items. Quality-based exclusion would therefore have resulted in including too few reviews in this work.
To conclude, AI shows a great potential to lead to accelerated, accurate, and more objective diagnoses of mental health disorders. The findings in this review strongly suggest that AI is on the jump into clinical use. Up-to-perfect performance is reported in many of the included studies, but much of that performance depends on the correct choice of data modality paired with correct technical choices (e.g., AI algorithms and methods). While AI promises a valid path for impartial and objective classification of mental disorders, practitioners in any field need to understand the basic aspect and behavior of their tools. We therefore believe that ethical considerations will gain importance in the future as well. With these considerations in mind, we recommend that healthcare professionals in the field (e.g., psychiatrists, psychologists) cautiously and consciously begin to explore the opportunities of AI-based tools for their daily routine. This recommendation is based on the potential we see in the technology reviewed in this study and the hope for rigorous evaluation in a clinical environment.
Methods
An umbrella review was conducted and reported in keeping with the Joanna Briggs Institute’s (JBI) guidelines for umbrella reviews43. The protocol for this review is registered at PROSPERO (ID: CRD42021231558).
Search strategy
We utilized the following bibliographic databases in our search: MEDLINE (via Ovid), PsycInfo (via EBSCO), CINAHL (EBSCO), IEEE Xplore, ACM Digital Library, Scopus, Cochrane Database of Systematic Reviews, DARE, and the PROSPERO register, JBI Evidence Synthesis, and Epistemonikos. These databases were searched on August 12, 2021 by the lead author. When applicable, we set auto alerts to conduct an automatic search weekly for 12 weeks (ending on December 12, 2021). We also searched the search engine “Google Scholar” to identify gray literature. We checked only the first 50 hits given that Google Scholar retrieved a massive number of hits and order them based on their relevancy. To identify further studies of relevance to the review, we screened the reference lists of included reviews (i.e., backward reference list checking) and identified and screened systematic reviews that cited the included reviews (i.e., forward reference list checking).
We developed the search query by consulting two experts in digital mental health and by checking systematic reviews of relevance to the review. These terms were chosen based on the target population (i.e., mental disorders), target intervention (i.e., AI-based approaches), and target study design (i.e., systematic review). Supplementary Table 2 presents the detailed search query used for searching each database.
Study eligibility criteria
This review included systematic reviews that focused on the performance of AI-based approaches in diagnosing mental disorders regardless of data type (e.g., neuroimaging data, neuropsychological data, demographical data, and clinical data), year of publication, and country of publication. We excluded systematic reviews that focused on AI-based approaches for predicting outcomes of intervention or prognosis of mental disorders. We also excluded reviews that did not show at least one of the following measures of classifier performance: accuracy, sensitivity, specificity, or area under the curve (AUC). Further, we excluded primary studies, scoping reviews, literature reviews, rapid reviews, criterial reviews, and other types of reviews. While systematic reviews published as journal articles, conference proceedings, and dissertations were included, we excluded conference abstracts and posters, commentaries, preprints, proposals, and editorials. We considered systematic reviews published only in the English language.
Study selection
We followed two steps to identify the relevant reviews. In the first step, two reviewers (AA and MH) independently checked the titles and abstracts of all identified studies. In the second step, the full texts of studies included from the first step were read by the two reviewers independently. In both steps, the two reviewers resolved any disagreements through discussion and consensus.
Data extraction
We developed a form to precisely and systematically extract the data from the included reviews (Supplementary Table 3). The form was pilot-tested using two included reviews. Two reviewers (AA & MH) independently extracted data from the included reviews using Microsoft Excel. Any disagreements between the reviewers were resolved through discussion and consensus.
Study quality appraisal
Two reviewers (AA and MH) independently assessed the quality of the included reviews using Joanna Briggs Institute Critical Appraisal Checklist for Systematic Reviews and Research Syntheses43. Any disagreements between the reviewers were resolved through discussion and consensus. Inter-rater agreement between the reviewers was very good (0.85)44.
Data synthesis
We synthesized the extracted data using the narrative approach. Specifically, results of the included reviews were grouped based on the target mental disorders that the AI classifiers distinguish. The results in each group were further aggregated based on the data types used to diagnose the target mental disorder. Given the high heterogeneity in the AI classifiers, data types, target mental disorder, and measures of classifier performance reported in the included reviews, we could not synthesize the results statistically. Therefore, we reported the range of results of measures of classifier performance. In addition, results that were reported by fewer than three primary studies in the included reviews are not reported in our review.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Su, C., Xu, Z., Pathak, J. & Wang, F. Deep learning in mental health outcome research: a scoping review. Transl. Psychiatry 10, 116 (2020).
Ohrnberger, J., Fichera, E. & Sutton, M. The relationship between physical and mental health: a mediation analysis. Soc. Sci. Med. 195, 42–49 (2017).
Rehm, J. & Shield, K. D. Global burden of disease and the impact of mental and addictive disorders. Curr. Psychiatry Rep. 21, 10 (2019).
Roland, J., Lawrance, E., Insel, T. & Christensen, H. The digital mental health revolution: transforming care through innovation and scale-up., (Doha, Qatar, 2020).
Bzdok, D. & Meyer-Lindenberg, A. Machine learning for precision psychiatry: opportunities and challenges. Biol. Psychiatry. Cogn. Neurosci. Neuroimaging 3, 223–230 (2018).
Roberts, L. W., Chan, S. & Torous, J. New tests, new tools: mobile and connected technologies in advancing psychiatric diagnosis. npj Digital Med. 1, 20176 (2018).
Abd-Alrazaq, A. et al. Artificial intelligence in the fight against COVID-19: scoping review. J. Med. Internet Res. 22, e20756 (2020).
Fiske, A., Henningsen, P. & Buyx, A. Your robot therapist will see you now: ethical implications of embodied artificial intelligence in psychiatry, psychology, and psychotherapy. J. Med Internet Res 21, e13216 (2019).
Góngora Alonso, S. et al. Social robots for people with aging and dementia: a systematic review of literature. Telemed. J. e-Health. Off. J. Am. Telemed. Assoc. 25, 533–540 (2019).
Martinez-Martin, N. & Kreitmair, K. Ethical issues for direct-to-consumer digital psychotherapy apps: addressing accountability, data protection, and consent. JMIR Ment. health 5, e32 (2018).
Torjesen, I. Sixty seconds on… sex with robots. BMJ 358, j3353 (2017).
Battista, P., Salvatore, C. & Castiglioni, I. Optimizing neuropsychological assessments for cognitive, behavioral, and functional impairment classification: a machine learning study. Behav. Neurol. 2017, 1850909 (2017).
Pinaya, W. H. L., Mechelli, A. & Sato, J. R. Using deep autoencoders to identify abnormal brain structural patterns in neuropsychiatric disorders: a large-scale multi-sample study. Hum. Brain Mapp. 40, 944–954 (2019).
Frangou, S., Dima, D. & Jogia, J. Towards person-centered neuroimaging markers for resilience and vulnerability in bipolar disorder. NeuroImage 145, 230–237 (2017).
Salminen, L. E. et al. Adaptive identification of cortical and subcortical imaging markers of early life stress and posttraumatic stress disorder. J. Neuroimaging Off. J. Am. Soc. Neuroimaging 29, 335–343 (2019).
Takagi, Y. et al. A neural marker of obsessive-compulsive disorder from whole-brain functional connectivity. Sci. Rep. 7, 7538 (2017).
Pellegrini, E. et al. Machine learning of neuroimaging for assisted diagnosis of cognitive impairment and dementia: a systematic review. Alzheimer’s Dement. Diagnosis Assess. Dis. Monit. 10, 519–535 (2018).
Billeci, L., Badolato, A., Bachi, L. & Tonacci, A. Machine learning for the classification of alzheimer’s disease and its prodromal stage using brain diffusion tensor imaging data: a systematic review. 8, https://doi.org/10.3390/pr8091071 (2020).
Sarica, A., Cerasa, A. & Quattrone, A. Random forest algorithm for the classification of neuroimaging data in Alzheimer’s disease: a systematic review. Front. Aging Neurosci. 9, 329 (2017).
Ebrahimighahnavieh, M. A., Luo, S. & Chiong, R. Deep learning to detect Alzheimer’s disease from neuroimaging: a systematic literature review. Computer Methods Prog. Biomedicine 187, 105242 (2020).
Petti, U., Baker, S. & Korhonen, A. A systematic literature review of automatic Alzheimer’s disease detection from speech and language. J. Am. Med. Inform. Assoc. 27, 1784–1797 (2020).
Battista, P. et al. Artificial intelligence and neuropsychological measures: the case of Alzheimer’s disease. Neurosci. Biobehav. Rev. 114, 211–228 (2020).
Law, Z. K. et al. The role of EEG in the diagnosis, prognosis and clinical correlations of dementia with lewy bodies-a systematic review. Diagnostics 10, 20 (2020).
de Filippis, R. et al. Machine learning techniques in a structural and functional MRI diagnostic approach in schizophrenia: a systematic review. Neuropsychiatr. Dis. Treat. 15, 1605–1627 (2019).
Steardo, L. Jr et al. Application of support vector machine on fMRI data as biomarkers in schizophrenia diagnosis: a systematic review. Front. psychiatry Front. Res. Found. 11, 588 (2020).
Bracher-Smith, M., Crawford, K. & Escott-Price, V. Machine learning for genetic prediction of psychiatric disorders: a systematic review. Mol. Psychiatry 26, 26 (2020).
Librenza-Garcia, D. et al. The impact of machine learning techniques in the study of bipolar disorder: a systematic review. Neurosci. Biobehav. Rev. 80, 538–554 (2017).
Moon, S. J. et al. Accuracy of machine learning algorithms for the diagnosis of autism spectrum disorder: systematic review and meta-analysis of brain magnetic resonance imaging studies. JMIR Ment. Health 6, e14108 (2019).
Ramos-Lima, L. F. et al. The use of machine learning techniques in trauma-related disorders: a systematic review. J. Psychiatr. Res. 121, 159–172 (2020).
Bruin, W., Denys, D. & van Wingen, G. Diagnostic neuroimaging markers of obsessive-compulsive disorder: Initial evidence from structural and functional MRI studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 91, 49–59 (2019).
Sanfelici, R., Dwyer, D. B., Antonucci, L. A. & Koutsouleris, N. Individualized diagnostic and prognostic models for patients with psychosis risk syndromes: a meta-analytic view on the state of the art. Biol. Psychiatry 88, 349–360 (2020).
American Psychological Association. Alzheimer’s disease, https://dictionary.apa.org/alzheimers-disease (2022).
American Psychological Association. Mild cognitive impairment (MCI), https://dictionary.apa.org/mild-cognitive-impairment (2022).
American Psychological Association. Schizophrenia, https://dictionary.apa.org/schizophrenia (2022).
American Psychological Association. Bipolar disorder, https://dictionary.apa.org/bipolar-disorders (2022).
American Psychological Association. Autism spectrum disorder https://dictionary.apa.org/autism-spectrum-disorder (2022).
American Psychological Association. Posttraumatic stress disorder https://dictionary.apa.org/posttraumatic-stress-disorder (2022).
American Psychological Association. Obsessive compulsive disorder, https://dictionary.apa.org/obsessive-compulsive-disorder (2022).
American Psychological Association. Psychotic disorders https://dictionary.apa.org/psychotic-disorders (2022).
Owen, M. J., Sawa, A. & Mortensen, P. B. Schizophrenia. Lancet 388, 86–97 (2016).
Robbins, J. If technology is a parasite masquerading as a symbiont—are we the host? IEEE Technol. Soc. Mag. 38, 24–33 (2019).
Sætra, H. S. The parasitic nature of social AI: sharing minds with the mindless. Integr. Psychol. Behav. Sci. 54, 308–326 (2020).
Aromataris, E. et al. Methodology for JBI umbrella reviews. 1–34 https://nursing.lsuhsc.edu/JBI/docs/ReviewersManuals/Umbrella%20Reviews.pdf (2014).
Altman, D. G. Practical statistics for medical research. (CRC press, 1990).
Acknowledgements
Open Access funding provided by the Qatar National Library.
Author information
Authors and Affiliations
Contributions
A.A.-a. developed the protocol and conducted the search with guidance from and under the supervision of M.H. Study selection and data extraction were carried out A.A.-a. & M.H. Risk of bias was assessed by A.A.-a. and C.T.T. A.A.-a. conducted data synthesis and wrote results and methods sections. D.A. wrote the introduction section. J.S., M.A. and A.A. wrote the discussion section. The article was revised critically for important intellectual content by all authors. All authors approved the manuscript for publication and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abd-alrazaq, A., Alhuwail, D., Schneider, J. et al. The performance of artificial intelligence-driven technologies in diagnosing mental disorders: an umbrella review. npj Digit. Med. 5, 87 (2022). https://doi.org/10.1038/s41746-022-00631-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41746-022-00631-8