Abstract
Sensor-based remote monitoring could help better track Parkinson’s disease (PD) progression, and measure patients’ response to putative disease-modifying therapeutic interventions. To be useful, the remotely-collected measurements should be valid, reliable, and sensitive to change, and people with PD must engage with the technology. We developed a smartwatch-based active assessment that enables unsupervised measurement of motor signs of PD. Participants with early-stage PD (N = 388, 64% men, average age 63) wore a smartwatch for a median of 390 days. Participants performed unsupervised motor tasks both in-clinic (once) and remotely (twice weekly for one year). Dropout rate was 5.4%. Median wear-time was 21.1 h/day, and 59% of per-protocol remote assessments were completed. Analytical validation was established for in-clinic measurements, which showed moderate-to-strong correlations with consensus MDS-UPDRS Part III ratings for rest tremor (⍴ = 0.70), bradykinesia (⍴ = −0.62), and gait (⍴ = −0.46). Test-retest reliability of remote measurements, aggregated monthly, was good-to-excellent (ICC = 0.75–0.96). Remote measurements were sensitive to the known effects of dopaminergic medication (on vs off Cohen’s d = 0.19–0.54). Of note, in-clinic assessments often did not reflect the patients’ typical status at home. This demonstrates the feasibility of smartwatch-based unsupervised active tests, and establishes the analytical validity of associated digital measurements. Weekly measurements provide a real-life distribution of disease severity, as it fluctuates longitudinally. Sensitivity to medication-induced change and improved reliability imply that these methods could help reduce sample sizes needed to demonstrate a response to therapeutic interventions or disease progression.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) affects 6 million people worldwide as of 2018—a number that is projected to grow to 12 million by 20401. Treatments are being developed to slow down or even halt the progression of PD2,3. However, currently used endpoints (e.g., the MDS-UPDRS) exhibit high within-subject variability, and low test-retest reliability, which leads to inefficient clinical trials, and risks potentially missing relevant effects4. Compounding this challenge, clinic-based physical exams provide only a snapshot of PD signs, and may not adequately reflect a patient’s functioning at home4,5. Additionally, many people live far from major medical centers6, so access to clinical trials of new therapeutics becomes restricted to a limited portion of the Parkinson population7,8.
These challenges have motivated the search for digital endpoints using wearable sensors, which allow for objective, frequent, and ecologically valid measurements of motor functioning in the patient’s home environment. Sensor-based remote monitoring could also help increase representation for groups whose data have historically not been included in clinical trials9,10. Before such measurements can be used as endpoints in clinical trials to quantify disease progression, a careful evaluation of the clinical validity, reliability, and sensitivity to change is required11,12.
A substantial volume of research has demonstrated the feasibility of using sensors placed on various parts of the body to quantify motor signs of PD13. Results suggest that features extracted by digital signal processing can be correlated with clinical outcomes of interest, at least when tests are delivered in a controlled setting and assessments are supervised by a clinician14,15,16,17,18,19,20,21,22. Active assessments measure patients’ maximum capacity, and can be complementary to passive monitoring, which measures the expression of signs in real life. Though some studies have probed the feasibility of using wearable sensors or smartphones for remote, self-guided active assessments, long-term engagement - which is critical to study disease progression - has been an important challenge23,24,25. Studies focusing on passive monitoring of PD motor signs have generally not been able to capture a person’s intent to move, which is particularly relevant for signs of bradykinesia26. Moreover, most of the existing work has focused on comparing sensor-based measures to clinical ratings, with limited work systematically measuring the ability of the remote measures to detect the effects of dopaminergic medication. Finally, test-retest reliability and sensitivity to clinically meaningful change have rarely been reported, and generally not on a large scale.
The smartwatch-based Parkinson’s Disease Virtual Motor Exam (PD-VME) can be deployed to remotely measure the severity of tremor, bradykinesia, and gait impairment, via a self-guided active assessment27. Here, we evaluate the feasibility of use and quality of data collected by the system, and report on the reliability, validity, and sensitivity to change of a set of digital measures derived from the PD-VME during a multi-year deployment in the Personalized Parkinson Project (PPP)27.
Results
Data were collected as part of the ongoing Personalized Parkinson Project (PPP), a prospective, longitudinal, single-center study (Clinical Trials NCT033648) of 520 people with early-stage Parkinson’s disease—diagnosed within the last 5 years27. Study participants wear a smartwatch (Verily Study Watch) for up to 23 h/day for the 3-year duration of the study, which passively collects raw sensor data from IMU, gyroscope, photoplethysmography, and skin conductance sensors.
Set 1 (N = 198 participants) was selected for video-based consensus scoring by matching age, gender, and MDS-UPDRS III score to be representative of the overall PPP study. Two assessors independently scored videos of the exams. When difficulties in rating MDS-UPDRS Part III tasks arose due to poor video quality, assessors provided scores only when confident in their assessment. MDS-UPDRS Part III consensus scores were computed as the median of the in-person rating and both video ratings.
Starting in May 2020, participants were offered the opportunity to enroll in a substudy, which asks them to perform an active assessment (Parkinson’s Disease Virtual Motor Exam, PD-VME) in the clinic and in remote, unsupervised settings. The PD-VME was deployed fully remotely, using digital instructions and an over-the-air firmware update to the watches of consented participants. A total of 370 participants enrolled in the substudy (Set 2).
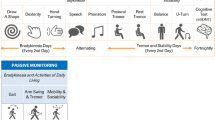
The smartwatch guides participants through the series of structured motor tasks comprising the PD-VME. It also allows patients on symptomatic medication to log the timing of their medication intake. The study design and patient-facing UI of the PD-VME are summarized in Fig. 1.
a Study design for the Personalized Parkinson’s Project. b Study schematic defining Sets 1 and 2 for the PD VME substudy. Set 1 consists of participants for whom consensus ratings of the MDS-UPDRS were available. Set 2 consists of participants who opted into the PD-VME substudy and performed at least one PD-VME. c User interface to prompt medication logging and PD-VME tasks. Seated rest, arm raise and arm twist (20 s duration), up-and-go (60 s duration).
Each week, participants were asked to perform the PD-VME twice on the same day, at two predefined times: first in the off state (selected as a time when they typically experienced their worst motor function), and then in the on-state (at a time when they typically experienced good motor function later in the day). Participants not taking medication were instructed to complete the PD-VME twice, one hour apart. The helpdesk at the site (Radboudumc) monitored wear-time and PD-VME completion and reached out to participants if more than three consecutive weekly assessments were missed.
Starting in July 2020, participants enrolled in the PD-VME substudy were asked to perform the PD-VME during their in-clinic visit (in the same manner as they did remotely), while the assessor observed its execution without providing feedback or any additional instructions. The in-clinic PD-VME is performed within 1 h after completion of the MDS-UPDRS part III off state exam, and before dopaminergic medication intake.
Demographic and clinical characteristics of the study population are presented in Table 1, for participants in Set 1 and Set 2. Distributions of the side on which the participants chose to wear the smartwatch are also included.
Engagement
Median smartwatch wear time across all PPP participants (N = 520)27,28 was 22.1 h/day, with a median follow-up period of 390 days. Variations in follow-up duration are due largely to the N = 126 who have not completed the study at the time of publication, and loss-to-follow-up is only 5.4%. Reasons for participant drop-out are indicated in Supplementary Table 2. Participants in Set 2 completed 22,668 PD-VMEs, corresponding to 59% of per-protocol test sessions during the 70-week follow-up period (Supplementary Fig. 1). In the first week, 80% of participants had at least 1 PD-VME, and 40% had completed one PD-VME in week 52.
Useability
Participants’ ability to perform the PD-VME was assessed during the in-clinic visit. Participants were able to complete the tasks in the exam (100% for tremor and upper-extremity bradykinesia and 98.5% for gait). Major protocol deviations were recorded as follows: participants did not place their hands on their lap during rest tremor tasks (8.2% of cases), participants performed the arm-twist using both arms (3.1% of cases), and participants either walked with their arms crossed across their chest (in 3.1% of cases) or sat down repeatedly (6.8% of cases) during the gait task. Detailed results are summarized in Supplementary Table 3.
Rest tremor
Among three measurements that were considered for measuring tremor severity, lateral tremor acceleration measurement is presented here because it showed the strongest correlation to in-clinic MDS-UPDRS ratings, and the strongest ability to separate on state from off state measurements. Results for additional measures are included in Supplementary Table 4.
The Spearman rank correlation between the median lateral acceleration during the rest tremor task and expert consensus rating of MDS-UPDRS task 3.17 was 0.70 [0.61, 0.77], N = 138 (Fig. 2a). For 56 participants, video quality was insufficient to ensure high confidence consensus ratings wrist acceleration signals intuitively map to the clinical observations during the MDS-UPDRS (Fig. 2b). Next, the sensitivity to on-off changes of the rest-tremor acceleration measurement was assessed (Fig. 2c). A small effect (Cohen’s d of 0.2) was observed comparing the on and off state. The mean difference in the measure was 0.10 [0.05, 0.1].
a Lateral tremor acceleration (log scale), measured during the in-clinic examination, by rest tremor (MDS-UPDRS 3.17) consensus score. Center lines: median, boxes: first and third quartiles, whiskers: 1.5x inter-quartile range. b Illustrative examples of raw lateral acceleration signals for each score on the MDS-UPDRS 3.17. Measurement values, as computed by the PD-VME, are also indicated. c Difference between the remote measurements in on and off states, aggregated over PD-VMEs obtained during the first two months from each participant. Mean and 95% confidence intervals across participants are represented. d Intra-class correlation (ICC) between at-home measurements, for various durations of aggregation. Whiskers represent 95% confidence intervals. The dotted blue line represents the published test-retest ICC of 0.79 for the whole rest tremor UPDRS Part III subcomponent (all four extremities + lip & jaw)44. e Distribution of PD-VME tremor measurements (off state) obtained during the in-clinic PD-VME (orange dot, representing a single measurement) and remote PD-VMEs (blue bar, representing the 25th to 75th percentile of PD-VMEs within 90 days of the in-clinic PD-VME), sorted on the remote PD-VMEs.
Test-retest reliability is reported in Fig. 2d, with intra-class correlation (ICC) of 0.71 [0.58–0.81] week-on-week (N = 208), and ICC of 0.90 [0.84–0.94] m s−2 for monthly averaged measures (N = 139).
Finally, the distribution of remote measurements compared to the sensor measurement during the in-clinic VME is shown in Fig. 2e. The in-clinic PD-VME measure was between the 25th and the 75th percentiles of the remote PD-VME measures for 41% of the participants.
Additional results for Postural tremor are included in Supplementary Table 5 and Supplementary Fig. 2.
Upper-extremity bradykinesia
Among the four measurements that were considered for measuring upper-extremity bradykinesia severity, no single measure showed both strong correlation to in-clinic MDS-UPDRS ratings, and a strong ability to separate on from off state measurements. Therefore, results are included below for both the arm-twist amplitude, and the arm-twist rate.
The highest correlation with expert consensus rating of MDS-UPDRS task 3.6 was observed for the arm twist amplitude measure, with ρ = −0.62 [−0.73, −0.49], N = 159 (Fig. 3a). However, the effect of medication state (Cohen’s d of −0.07) was very small (Fig. 3c)29. The mean on-off difference in the measure was −0.9 [0.0, −1.6] degrees. Test-retest ICC (Fig. 3d) was 0.71 [0.59–0.80] week-on-week (N = 208) and 0.89 [0.84–0.94] for monthly-averaged measures (N = 136). The in-clinic PD-VME measure was between the 25th and the 75th percentiles of the remote PD-VME measures for 45% of the participants.
a Arm twist amplitude measured during the in-clinic examination, by pronation-supination (MDS-UPDRS 3.6) consensus scores. Center lines: median, boxes: first and third quartiles, whiskers: 1.5x inter-quartile range. b Illustrative examples of raw gyroscope signals, along the x axis, for each score on the MDS-UPDRS 3.6. Measurement values, as computed by the PD-VME, are also indicated. c Difference between the remote measurements in on and off states, aggregated over PD-VMEs obtained during the first two months from each participant. Mean and 95% confidence intervals across participants are represented. d Intra-class correlation (ICC) between at-home measurements, for various durations of aggregation. Whiskers represent 95% confidence intervals. The dotted blue line represents the published test-retest ICC of 0.89 for the whole bradykinesia subcomponent of the UPDRS Part III31. e Distribution of PD-VME arm-twist measurements (off state) obtained during the in-clinic PD-VME (orange dot, representing a single measurement) and remote PD-VMEs (blue bar, representing the 25th to 75th percentile of PD-VMEs within 90 days of the in-clinic PD-VME), sorted on the remote PD-VMEs.
The assessors observed during the in-clinic PD-VME exam that some patients mainly focussed on the speed of the arm-twist movement rather than the amplitude. Therefore, sensor-based measures of the rate of arm-twist and the combination of rate and amplitude were investigated as well. Correlations to the consensus MDS-UPDRS ratings of ρ = 0.06 [−0.25, +0.13] for arm-twist rate, and ρ = −0.42 [−0.55, −0.28] for the product of rate and amplitude were observed. Both metrics showed significant change in on and off: Cohen’s d of −0.22 and mean change of −0.16 [−0.13, −0.20] s−1 for arm-twist rate, and Cohen’s d of −0.26 and mean change of −8 [−6, −10] degrees/s for the combination. The full results are included in Supplementary Table 6.
Arm swing during gait
Among the three measurements that were considered for measuring gait impairment, arm swing acceleration was selected. While it was not the best outcome measure across any of the criteria, it showed solid performance across all of them. Results for the measures that were not selected are included in Supplementary Table 7.
The Spearman rank correlation between the arm swing acceleration during the gait task and expert consensus rating of MDS-UPDRS task 3.10 was ρ = −0.46 [−0.58, −0.31], N = 164 (Fig. 4a). A small effect (Cohen’s d of 0.44) was observed comparing the on and off state. The mean difference in the measure was −0.8 [−1.2, −0.5] m−s−2. Test-retest ICC (Fig. 4d) was 0.43 [0.30–0.56] week-on-week (N = 210), and 0.75 [0.66–0.84] for monthly-averaged measures (N = 139). The in-clinic PD-VME measure was between the 25th and the 75th percentiles of the remote PD-VME measures for 39% of the participants.
a Arm swing acceleration measured during the in-clinic examination, separated by gait (MDS-UPDRS 3.10) consensus scores. Center lines: median, boxes: first and third quartiles, whiskers: 1.5x inter-quartile range. b Illustrative examples of raw accelerometer signals, for each score on the MDS-UPDRS 3.10. Measurement values, as computed by the PD-VME, are also indicated. c Difference between the remote measurements in on and off states, aggregated over PD-VMEs obtained during the first two months from each participant. Mean and 95% confidence intervals across participants are represented. d Intra-class correlation (ICC) between at-home measurements, for various durations of aggregation. Whiskers represent 95% confidence intervals44. e Distribution of PD-VME gait measurements (off state) obtained during the in-clinic PD-VME (orange dot, representing a single measurement) and remote PD-VMEs (blue bar, representing the 25th to 75th percentile of PD-VMEs within 90 days of the in-clinic PD-VME), sorted on the at-home PD-VMEs.
Discussion
The data from this study suggest that people with PD engage with and are able to use the PD-VME, and that the quality of data collected is high enough to enable evaluation of the analytical validity, reliability, and sensitivity to change of digital measures built from this system.
A digital solution is only useful if people with PD engage with it regularly. We observed robust levels of engagement, both in terms of overall wear time (>21 h/day) and engagement with the active assessment, which was 59% over one year when assayed on a weekly basis. This is at the high end of reported values23,24, and this suggests that combining active assessments with passive monitoring on smartwatch form-factors have the potential to yield substantial quantities of high-quality data. For studies assessing longitudinal progression, our observations suggest that even higher engagement could be obtained by requiring a set of weekly unsupervised tests for a limited duration at baseline and again at the end of the follow-up period.
We showed a moderate-to-strong correlation between in-clinic MDS-UPDRS Part III measurements and consensus clinical ratings for rest tremor, bradykinesia, and arm swing during gait, which provided analytical validation of the individual measurements. These results are on par with similar published analyses of wrist-worn sensors30,31,32,33 and demonstrate the ability of the PD-VME to provide metrics that map to the observations of an expert clinician. While the moderate-to-strong correlations with MDS-UPDRS scores establish that the measurements are working as intended, engineering for perfect correlation simply recreates an imperfect scoring system, and washes out the potential for increased sensitivity of sensor-based measurements. One key reason for making a shift towards digital assessments is that clinical scores remain subjective in nature, and use a low resolution, ordinal scoring system. The criteria for transitioning between different scores leave much room for subjective interpretation, and cause considerable variability between and within raters in daily practice4.
This is exemplified by the results shown for the upper-extremity bradykinesia measure, in which we find that the measure most correlated with in-clinic MDS-UPDRS ratings - amplitude of arm-twist - is not the one that is most sensitive to change from dopaminergic medication. It is possible that while the experts are instructed to evaluate “speed, amplitude, hesitations, halts and decrementing amplitude34”, they may focus mostly on amplitude. Similarly, we observe a gradient of tremor measurements, both in-clinic and remotely, even within the group of participants who are rated as a 0 on the MDS-UPDRS 3.15 or 3.17. This suggests that some amount of tremor could be present, both in the clinic and at-home, even before it becomes apparent to the human eye. Indeed, it is generally a well-accepted phenomenon that tremors are easier felt or even heard (using a stethoscope) than observed by an examiner. This reinforces the need for objective sensor-based measures, and the need to evaluate these measures based on their ability to detect clinically meaningful changes rather than simply reproducing subjective clinical exams.
In people with PD, dopaminergic medication can considerably improve severity of motor signs over short time frames. This “on-off” difference is well-accepted as a clinically meaningful change, and when coupled with wearable sensors and patient-reported tagging of daily medication regimen, creates multiple “natural experiments” in the course of patients’ daily lives. These allow us to test the clinical validity35,36 of the PD-VME measures as pharmacodynamic/response biomarkers for people with PD in the remote setting. Indeed, we demonstrate that digital measures for tremor, upper-extremity bradykinesia and gait are able to detect significant change in patients’ motor signs before and after medication intake.
For clinical trials aiming to show disease modification, measurements that provide reliable estimates of a subject’s disease state can increase statistical power, and reduce the required sample size or trial duration. However, measuring long-term progression using infrequent measurements is difficult, because motor and non-motor signs of PD can markedly fluctuate from moment to moment31,37, depending on factors such as the timing of medication intake or the presence of external stressors. The increased test-retest reliability of the monthly aggregated measures from this study suggest that collecting outcome measures remotely and at an increased frequency increases their reliability, and has the potential to measure progression of the average motor sign severity.
This work is not without its limitations. The smartwatch was worn unilaterally, though PD typically exhibits asymmetrical symptom severity. Asymmetry may have particularly affected our assessments of gait which may exhibit different characteristics when observed from the most- or least-affected upper-extremity. Further analysis is needed to better understand the impact of device placement on measurement validity and reliability. Also, PD is multifaceted in nature, and signs manifest along multiple motor and non-motor domains38. While we present data on multiple important motor domains, additional research is needed to use the rich data collected in this study to expand this to additional motor and non-motor aspects (e.g., by using the PPG and EDA data collected). Finally, future work replicating the results presented here in a different study population is needed. In particular, a study of people with more advanced PD could enable further understanding of the impact of medication-induced dyskinesia on the sensor-based measurements. In addition to enabling exploratory analyses around non-motor symptoms, the 3-year follow-up of PPP will enable future work looking at the PD-VME’s sensitivity to long-term disease progression. The smart-watch form-factor could enable future analysis combining the PD-VME measurements together with measures obtained from passive monitoring. For signs of bradykinesia in particular, active tasks allow us to capture the intent to move, which may be difficult to capture from passively collected data.
In conclusion, these data suggest that patients engage robustly with the PD-VME, and are able to complete remote active assessments of motor function to yield data of a sufficient quality to generate digital measurements of motor signs, test their analytical validity, and assess their sensitivity to change in medication status. The system allows for an increased frequency of data collection, enabling monthly aggregation of measurements, leading to increased test-retest reliability. In turn, high reliability suggests that these measures have potential as digital biomarkers of progression. Further research is needed to more firmly establish the ability of these and other measures to serve as progression biomarkers.
Methods
Study design
To maximize engagement, participants were instructed to consistently wear the watch on the side that is most convenient for them. All sensor data collected in this study used a wrist-worn wearable device, the 2nd-generation of the Verily Study Watch (hardware and firmware characteristics can be found in the supplementary materials).
Inclusion criteria for this study include: PD diagnosed in the last 5 years; 18 years of age or older;
Ability to provide written informed consent; no nickel allergy (as components of the Study Watch contain this metal). Patients with co-morbidity are explicitly NOT excluded from participation. There were no inclusion/exclusion criteria based on participants’ familiarity or comfort with technology.
Sensor data is collected during the yearly in-clinic MDS-UPDRS Part III motor exams. These are conducted in both the on and off states, after overnight withdrawal of dopaminergic medication (at least 12 h after the last intake). Exams are video-recorded for quality controls and offline consensus scoring. The datasets that were used for the analysis are described in Table 1 and the Results section.
Design of the Parkinson’s disease virtual motor exam
The PD-VME system, including participant-facing training materials, user interface, task choice and digital measures, was developed using a patient-centric approach39. The PD-VME comprises eight tasks designed to assess various domains of motor signs: rest and postural tremor, upper extremity bradykinesia through finger tapping, pronation-supination and repeated hand opening and closing, lower-extremity bradykinesia through foot stomping, gait and postural sway. Participant-facing instructions for each task are in Supplementary Table 1. Figure 1c shows the PD-VME user interface for the four tasks analyzed in this paper. Selection of targeted signs was informed by research on meaningful aspects of health in PD: tremor, bradykinesia and gait were identified as three of the top four symptoms that people with PD most want to improve40. A patient panel of PPP participants was involved throughout the design process to assess and improve the usability of the system.
During execution of PD-VME tasks, tri-axial accelerometer and gyroscope data was collected at a sample rate of 200 Hz. For each task, an initial list of concepts of interest were identified (e.g., tremor severity, quality of gait). For each concept, digital signal processing was implemented to convert the raw sensor data into 11 exploratory outcome measures (e.g., tremor acceleration, arm-swing magnitude).
Evaluation of digital measures from the PD-VME
Participant engagement with the PD-VME, measured as the fraction of participants who performed at least one complete exam in a given week, was evaluated over the course of 70 weeks. The ability of the participants to perform the PD-VME correctly without having received in-person instructions was assessed using the assessor observations from the in-clinic PD-VME.
The analytical validity, reliability, and sensitivity to change of digital measurements from the PD-VME was evaluated. First, the analytical validity of measures, collected during the in-clinic MDS-UPDRS, was assessed using the Spearman correlation coefficient of the measure against the consensus of three independent MDS-UPDRS Part III clinical ratings. Second, the test-retest reliability in the home setting was evaluated by computing the intra-class correlation between monthly means across subsequent months for months with no missing PD-VME. Finally, the sensitivity to change was assessed by testing the ability of the remote measurements to distinguish between the off and the on states for the subset of patients in Set 2 who are on dopaminergic medication. An unsupervised PD-VME exam is determined to be in the off state if it occurred at the pre-scheduled off time and at least 6 h after a medication tag. Similarly, an exam is determined to be in the on state if it occurred at the pre-scheduled on time and between 0.5 and 4 h after a medication tag. Two measures were used to assess the magnitude of change: mean difference (and associated 95% confidence interval) and Cohen’s d. Participants taking dopamine agonists were not included in the on-off comparison because of their prolonged effect.
For each task, one outcome measure is shown in the results, selected on the basis of its high performance across all three aspects (analytical validity, test-retest reliability, and sensitivity to change) for inclusion in the results. The results of the remaining 8 measures are presented in the supplementary materials.
Comparison of the in-clinic and remotely collected PD-VME measurements
To characterize the extent to which measures obtained from clinic-based physical exams (off) reflected patients’ signs in the remote setting (off), the distributions of participants’ in-clinic and remote PD-VME outcomes (completed within 90 days of the clinic visit) were compared. A subset of N = 194 participants from Set 2 who performed the PD-VME in-clinic was included in this analysis.
Figures and statistical analyses were generated using the Python programming language, using the SciPy41, Matplotlib42 and seaborn43 libraries. In all numerical results that follow, point estimates are followed by 95% confidence intervals in square brackets. Confidence intervals were calculated using the bootstrap method with 1000 resampling iterations.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The datasets generated during this study will be made available to qualified researchers worldwide, insofar as such requests are for analysis for which the participants in the PPP have provided informed consent. Requests for access to data are governed by a Research and Data Sharing Review Committee (RDSRC), whose role is to protect subjects’ privacy by limiting the availability of the study data and controlling access to sources of information that might potentially be used to identify the individual subjects associated with the biospecimen analyses. The RDSRC will assess the relevance and scientific quality of research proposals for which study data or material is requested27. Inquiries regarding access to the data can be sent to info@parkinsonopmaat.nl.
Code availability
The code used in the study is proprietary, and will not be made available. We direct readers to the data availability section on applications for data use as a qualified researcher.
Change history
28 December 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41746-022-00744-0
References
Dorsey, E. R., Ray Dorsey, E., Sherer, T., Okun, M. S. & Bloem, B. R. The emerging evidence of the Parkinson pandemic. J. Parkinson’s Dis. 8, S3–S8 (2018).
Lang, A. E. & Espay, A. J. Disease modification in Parkinson’s disease: Current approaches, challenges, and future considerations. Mov. Disord. 33, 660–677 (2018).
McFarthing, K. et al. Parkinson’s disease drug therapies in the clinical trial pipeline: 2020. J. Parkinsons. Dis. 10, 757–774 (2020).
Evers, L. J. W., Krijthe, J. H., Meinders, M. J., Bloem, B. R. & Heskes, T. M. Measuring Parkinson’s disease over time: The real-world within-subject reliability of the MDS-UPDRS. Mov. Disord. 34, 1480–1487 (2019).
Czech, M. D. et al. Age and environment-related differences in gait in healthy adults using wearables. NPJ Digit Med 3, 127 (2020).
Galsky, M. D. et al. Geographic accessibility to clinical trials for advanced cancer in the United States. JAMA Intern. Med. 175, 293 (2015).
Feyman, Y., Provenzano, F. & David, F. S. Disparities in clinical trial access across US Urban Areas. JAMA Netw. Open 3, e200172 (2020).
Clougherty, J. E. et al. Geography, generalisability, and susceptibility in clinical trials. Lancet Respir. Med 9, 330–332 (2021).
Izmailova, E. S., Ellis, R. & Benko, C. Remote monitoring in clinical trials during the COVID-19 pandemic. Clin. Transl. Sci. 13, 838–841 (2020).
Motolese, F. et al. Parkinson’s disease remote patient monitoring during the COVID-19 lockdown. Front. Neurol. 11, 567413 (2020).
Manta, C., Patrick-Lake, B. & Goldsack, J. C. Digital measures that matter to patients: A framework to guide the selection and development of digital measures of health. Digit Biomark. 4, 69–77 (2020).
Stephenson, D., Badawy, R., Mathur, S., Tome, M. & Rochester, L. Digital progression biomarkers as novel endpoints in clinical trials: A multistakeholder perspective. J. Parkinsons. Dis. 11, S103–S109 (2021).
Thorp, J. E., Adamczyk, P. G., Ploeg, H.-L. & Pickett, K. A. Monitoring motor symptoms during activities of daily living in individuals with Parkinson’s disease. Front. Neurol. 9, 1036 (2018).
Corrà, M. F. et al. Comparison of laboratory and daily-life gait speed assessment during ON and OFF states in Parkinson’s disease. Sensors 21, (2021).
Mahadevan, N. et al. Development of digital biomarkers for resting tremor and bradykinesia using a wrist-worn wearable device. NPJ. Digit. Med. 3, 5 (2020).
Del Din, S. et al. Gait analysis with wearables predicts conversion to parkinson disease. Ann. Neurol. 86, 357–367 (2019).
Zhan, A. et al. Using smartphones and machine learning to quantify Parkinson disease severity: The mobile Parkinson disease score. JAMA Neurol. 75, 876–880 (2018).
Lonini, L. et al. Wearable sensors for Parkinson’s disease: Which data are worth collecting for training symptom detection models. NPJ Digit. Med. 1, 64 (2018).
Arora, S. et al. Detecting and monitoring the symptoms of Parkinson’s disease using smartphones: A pilot study. Parkinsonism Relat. Disord. 21, 650–653 (2015).
Mirelman, A. et al. Arm swing as a potential new prodromal marker of Parkinson’s disease. Mov. Disord. 31, 1527–1534 (2016).
Williamson, J. R., Telfer, B., Mullany, R. & Friedl, K. E. Detecting Parkinson’s disease from wrist-worn accelerometry in the U.K. Biobank. Sensors 21, 2047 (2021).
Braybrook, M. et al. An ambulatory tremor score for Parkinson’s disease. J. Parkinsons. Dis. 6, 723–731 (2016).
Lipsmeier, F. et al. Evaluation of smartphone-based testing to generate exploratory outcome measures in a phase 1 Parkinson’s disease clinical trial. Mov. Disord. 33, 1287–1297 (2018).
Bot, B. M. et al. The mPower study, Parkinson disease mobile data collected using ResearchKit. Sci. Data 3, 160011 (2016).
WATCH-PD: Wearable Assessments in the Clinic and Home in Parkinson’s Disease: Baseline Analyses—MDS Abstracts. https://www.mdsabstracts.org/abstract/watch-pd-wearable-assessments-in-the-clinic-and-home-in-parkinsons-disease-baseline-analyses/ (2021).
Powers, R. et al. Smartwatch inertial sensors continuously monitor real-world motor fluctuations in Parkinson’s disease. Sci. Transl. Med. (2021) https://doi.org/10.1126/scitranslmed.abd7865.
Bloem, B. R. et al. The Personalized Parkinson Project: examining disease progression through broad biomarkers in early Parkinson’s disease. BMC Neurol. 19, 160 (2019).
McDermott, M. M. & Newman, A. B. Remote research and clinical trial integrity during and after the Coronavirus pandemic. JAMA 325, 1935–1936 (2021).
Sawilowsky, S. S. New effect size rules of thumb. J. Mod. Appl. Stat. Methods 8, 597–599 (2009).
Zampieri, C. et al. The instrumented timed up and go test: potential outcome measure for disease modifying therapies in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 81, 171–176 (2010).
Haubenberger, D. et al. Transducer-based evaluation of tremor. Mov. Disord. 31, 1327–1336 (2016).
Dirkx, M. F., Zach, H., Bloem, B. R., Hallett, M. & Helmich, R. C. The nature of postural tremor in Parkinson disease. Neurology 90, e1095–e1103 (2018).
Elble, R. J. et al. Tremor amplitude is logarithmically related to 4- and 5-point tremor rating scales. Brain 129, 2660–2666 (2006).
Goetz, C. G. et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 23, 2129–2170 (2008).
Goldsack, J. C. et al. Verification, analytical validation, and clinical validation (V3): the foundation of determining fit-for-purpose for Biometric Monitoring Technologies (BioMeTs). NPJ Digit Med 3, 55 (2020).
FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource. (Food and Drug Administration (US), 2016).
Zach, H., Dirkx, M., Bloem, B. R. & Helmich, R. C. The clinical evaluation of Parkinson’s tremor. J. Parkinson’s Dis. 5, 471–474 (2015).
Stebbins, G. T., Goetz, C. G., Lang, A. E. & Cubo, E. Factor analysis of the motor section of the unified Parkinson’s disease rating scale during the off-state. Mov. Disord. 14, 585–589 (1999).
Taylor, K. I., Staunton, H., Lipsmeier, F., Nobbs, D. & Lindemann, M. Outcome measures based on digital health technology sensor data: data- and patient-centric approaches. NPJ Digit Med 3, 97 (2020).
Port, R. J. et al. People with Parkinson’s disease: What symptoms do they most want to improve and how does this change with disease duration? J. Parkinsons. Dis. 11, 715–724 (2021).
Jones, E., Oliphant, T. & Peterson, P. SciPy: Open Source Scientific Tools for Python (2001). http://www.scipy.org/.
Hunter, J. D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 9, 90–95 (2007).
Waskom, M. seaborn: statistical data visualization. J. Open Source Softw. 6, 3021 (2021).
Siderowf, A. et al. Test-retest reliability of the unified Parkinson’s disease rating scale in patients with early Parkinson’s disease: Results from a multicenter clinical trial. Mov. Disord. 17, 758–763 (2002).
Acknowledgements
We are grateful to the Personalized Parkinson’s Project participants who shared their time interacting with the PD-VME, provided invaluable feedback on the design and user experience, and shared their experience with PD. For their contributions to the study design, study operations, and data collection, we would like to recognize Cindy Yee, Anisha Choudhury, Kinjal Satasiya, Nicole Greco, Tessa van de Zande, Myrthe Burgler, Marjan Meinders, Sabine van Zundert, Debbie de Graaf, and Ginny Snellen. For reviewing and providing feedback on the manuscript, we would like to acknowledge: Nate Kowahl, Sooyoon Shin, Li-Fang Cheng, Niranjan Sridhar, Zachary Owen and Sara Popham.
Author information
Authors and Affiliations
Contributions
Conceptualization: M.B., R.K., W.J.M., B.R.B., E.R., L.M. Methodology: M.B., R.K., E.R., B.R.B. Software: M.B., K.C.H., E.R. Validation: M.B., K.C.H., C.C., B.R.B. Formal Analysis: M.B., K.C.H., E.R., B.R.B. Investigation: B.R.B., R.K. Resources: B.R.B., R.K. Data Curation: M.B., E.R., K.C.H., B.R.B. Writing—original draft: M.B., E.R., K.C.H., C.C., R.K. Writing—review & editing: B.R.B., W.J.M., L.E., R.H., L.M. Visualization: K.C.H., M.B., E.R., R.K. Funding acquisition: R.K., W.J.M., B.R.B. Supervision: R.K., L.M., B.R.B. Project Administration: B.R.B., R.K.
Corresponding author
Ethics declarations
Competing Interests
All authors with Verily affiliation are current Verily employees and are Verily shareholders. The study is financially supported by Verily Life Sciences LLC, Radboud University Medical Center, Radboud University, the city of Nijmegen, and the Province of Gelderland. Radboud UMC received funding for the original cohort from Health Holland (PPP allowance; public-private partnership), Radboud University Radboudumc, and Radboudumc (in kind). The other study sponsors had no role in study design and collection, analysis and interpretation of data, or in writing the manuscript. Prof. Bloem serves as the co-Editor in Chief for the Journal of Parkinson’s disease, serves on the editorial board of Practical Neurology and Digital Biomarkers, has received fees from serving on the scientific advisory board for UCB, Kyowa Kirin, Zambon, and the Critical Path Institute (paid to the. Institute), has received fees for speaking at conferences from AbbVie, Biogen, UCB, Zambon, Roche, GE Healthcare, Oruen and Bial (paid to the Institute), and has received research support from the Netherlands Organization for Health Research and Development, the Michael J Fox Foundation, UCB, the Stichting Parkinson Fonds, Hersenstichting Nederland, de Stichting Woelse Waard, Stichting Alkemade-Keuls, de Maag Lever Darm Stichting, Parkinson NL, Davis Phinney Foundation, the Parkinson’s Foundation, Verily Life Sciences, Horizon 2020, the Topsector Life Sciences and Health, Nothing Impossible and the Parkinson Vereniging, outside the submitted work. Prof. Bloem does not hold any stocks or stock options with any companies that are connected to Parkinson’s disease or to any of the topics in this paper. Prof. Bloem has received grants/research support from Netherlands Organization for Scientific Research, the Michael J Fox Foundation, Nothing Impossible, Parkinson Vereniging, Parkinson’s Foundation, Hersenstichting Nederland, Davis Phinney Foundation, Parkinson NL, Stichting Woelse Waard, Stichting Alkemade-Keuls, Maag Lever Darm Stichting, Verily Life Sciences, Horizon 2020, Topsector Life Sciences and Health, UCB, Zambon. Prof. Bloem has been a consultant for the Critical Path Institute, Kyowa Kirin, UCB, Zambon, and has received speaking fees from AbbVie, Bial, Biogen, GE Healthcare, Oruen, Roche, UCB, Zambon. The remaining authors declare no competing interests.
Ethics
The study is being conducted in conformance with the Good Clinical Practice ICH E6 guideline and in compliance with the Ethical Principles for Medical Research Involving Human Subjects, as defined in the Declaration of Helsinki (version amended in October 2013), the Dutch Personal Data Protection Act, and the European General Data Protection Regulation. The Commissie Mensgebonden Onderzoek Region Arnhem-Nijmegen approved the study protocol and communication materials27. Signed informed consent is obtained before engaging a participant in any study procedure, including use of the PD-VME.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Burq, M., Rainaldi, E., Ho, K.C. et al. Virtual exam for Parkinson’s disease enables frequent and reliable remote measurements of motor function. npj Digit. Med. 5, 65 (2022). https://doi.org/10.1038/s41746-022-00607-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41746-022-00607-8
This article is cited by
-
Upper limb intention tremor assessment: opportunities and challenges in wearable technology
Journal of NeuroEngineering and Rehabilitation (2024)
-
Improved measurement of disease progression in people living with early Parkinson’s disease using digital health technologies
Communications Medicine (2024)
-
Attitudes Toward the Adoption of Remote Patient Monitoring and Artificial Intelligence in Parkinson’s Disease Management: Perspectives of Patients and Neurologists
The Patient - Patient-Centered Outcomes Research (2024)
-
Evidence from ClinicalTrials.gov on the growth of Digital Health Technologies in neurology trials
npj Digital Medicine (2023)
-
Application of single wrist-wearable accelerometry for objective motor diary assessment in fluctuating Parkinson’s disease
npj Digital Medicine (2023)