Abstract
Consumer wearables and sensors are a rich source of data about patients’ daily disease and symptom burden, particularly in the case of movement disorders like Parkinson’s disease (PD). However, interpreting these complex data into so-called digital biomarkers requires complicated analytical approaches, and validating these biomarkers requires sufficient data and unbiased evaluation methods. Here we describe the use of crowdsourcing to specifically evaluate and benchmark features derived from accelerometer and gyroscope data in two different datasets to predict the presence of PD and severity of three PD symptoms: tremor, dyskinesia, and bradykinesia. Forty teams from around the world submitted features, and achieved drastically improved predictive performance for PD status (best AUROC = 0.87), as well as tremor- (best AUPR = 0.75), dyskinesia- (best AUPR = 0.48) and bradykinesia-severity (best AUPR = 0.95).
Similar content being viewed by others
Introduction
Digital measurements provided through clinical and consumer devices such as wearables, phones, and smartwatches are providing opportunities to monitor the disease, treatment effects, and the daily lived experience of disease through the collection of real-world evidence1. While most efforts to incorporate these types of data have been in the context of exploratory and feasibility studies, we are increasingly seeing evidence of their use as digital endpoints in clinical trials2. Interpretation of the data streams from these devices into sensitive ‘digital biomarkers’ and endpoints requires the development of sophisticated analytical algorithms, and vetting these algorithms requires extensive validation against quality datasets, using unbiased evaluation methods. Here we describe the use of an unbiased approach to benchmark multiple approaches for deriving clinically relevant features of disease through crowdsourcing and independent evaluation.
One area of emerging digital biomarker development is Parkinson’s disease (PD), a neurodegenerative disorder that conspicuously affects motor function, along with other domains such as cognition, mood, and sleep. Classic motor symptoms of the disease include tremors, slowness of movement (bradykinesia), posture and gait perturbations, impaired coordination and muscle rigidity, which can affect a patients’ ability to function in daily life. Parkinson’s symptoms usually start gradually but get more severe and usually lead to medical intervention to relieve symptoms. As PD is exemplified by low brain dopamine levels, one of the primary pharmacologic treatments for PD involves the use of synthetic dopamine or dopamine agonists, such as levodopa. Some patients exhibit motor side effects of medication, chiefly involuntary movements, known as dyskinesia, which themselves can also be disruptive to patients. The strong motor symptom component of the disease and treatment side-effects makes PD ideally suited to monitoring with motion sensors such as accelerometers and gyroscopes to understand the frequency and severity of these symptoms in the patients’ daily life in order to optimally treat them. Multiple approaches have leveraged accelerometer and gyroscope data from wearable devices for the development of digital biomarkers in PD (see for example3,4). However, they have yet to be translated into clinical care as outcome measures or as primary biomarkers in clinical trials.
The primary barrier to the incorporation of digital biomarkers in clinical or regulatory settings, is the (deservedly) high bar for validation of these complex algorithms, to show both the accuracy and optimality of the measure. Unfortunately, validation work is both expensive and difficult to perform, leading to often underpowered validation studies evaluated by a single research group and, hence, subject to the self-assessment trap5. Pre-competitive efforts are underway such as Critical Path’s Patient Reported Outcome (PRO) Consortium6 and the open wearables initiative (OWI). Here we describe an open initiative to both competitively and collaboratively evaluate analytical approaches for the estimation of PD severity in an unbiased manner.
The Parkinson’s Disease Digital Biomarker (PDDB) DREAM Challenge (https://www.synapse.org/DigitalBiomarkerChallenge) benchmarked crowd-sourced methods of processing sensor data (i.e., feature extraction), which can be used in the development of digital biomarkers that are diagnostic of disease or can be used to assess symptom severity. In short, the PDDB Challenge participants were provided with training data, which included sensor data, as well as disease status or symptom severity labels. They were also provided a separate test set, which contained sensor data only. Given raw sensor data from two studies, participating teams engineered features from the sensor data that were evaluated on their ability to predict disease labels in models built using an ensemble-based predictive modeling pipeline.
The challenge leveraged two different datasets. In order to assess the ability to predict whether an individual has PD, we used mPower7, a remote smartphone-based study. While a portion of the mPower dataset had previously been released publicly, a second portion of the data remained private to the challenge organizers. This allowed challenge evaluations to be performed in a blinded, unbiased manner. In this study, accelerometer and gyroscope data from a gait and balance (walking/standing) test in 4799 individuals (76,039 total measures) were provided to participants in order to engineer features to discriminate patients with PD from controls.
In order to assess the ability to predict symptom severity, we used the Levodopa (L-dopa) Response Study8,9, a multi-wearable clinical study that included symptom severity assessment by trained clinicians. This dataset had not been shared publicly at the time of the challenge. In this study, accelerometer recordings from GENEActiv and Pebble watches were captured on two separate days from 25 patients exhibiting motor fluctuations10 (i.e., the side effects and return of symptoms after administration of levodopa), as they were evaluated for symptom severity during the execution of short, 30 s, motor tasks designed to evaluate tremor, bradykinesia, and dyskinesia. Data collection during the battery of tasks was repeated six to eight times over the course of each day in 30 min blocks, resulting in 3–4 h motor activity profiles reflecting changes in symptom severity. In total 8239 evaluations were collected across three different PD symptoms.
Results
The Parkinson’s Disease Digital Biomarker DREAM Challenge
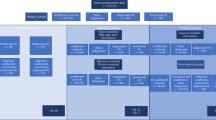
We developed four sub-challenges using the two datasets; one using data from the mPower Study and three using data from the L-dopa Response Study. Using the mPower data, we sought to determine whether mobile sensor data from a walking/standing test could be used to predict PD status (based on a professional diagnosis as self-reported by the study subjects) relative to age-matched controls from the mPower cohort (sub-challenge 1 (SC1)) (Supplementary Table 1). The three remaining sub-challenges used the L-dopa data to predict symptom severity as measured by: active limb tremor severity (0–4 range) using mobile sensor data from six bilateral upper-limb activities (sub-challenge 2.1 (SC2.1)); resting upper-limb dyskinesia (presence/absence) using bilateral measurements of the resting limb while patients were performing tasks with the alternate arm (sub-challenge 2.2 (SC2.2)); and presence/absence of active limb bradykinesia using data from five bilateral upper-limb activities (sub-challenge 2.3 (SC2.3)). Participants were asked to extract features from the mobile sensor data, which were scored using a standard set of algorithms for their ability to predict the disease or symptom severity outcome (Fig. 1).
For each sub-challenge, data were split into training and test portions. Participants were provided with the mobile sensor data for both the training and test portions, along with the demographic (SC1 only) and meta-data, and diagnosis or severity labels for the training portion of the data only. Participants were asked to derive features from the mobile sensor data for both the training and test portions of the data. These features were then used to train a classifier, using a standard suite of algorithms, to predict disease status or symptom severity, and predict labels in the testing portion of the data. Submissions were scored based on the accuracy of the resulting predictions.
For SC1, we received 36 submissions from 20 unique teams, which were scored using the area under the ROC curve (AUROC) (see methods). For comparison, we also fit a ‘demographic’ baseline model, which included only age and gender. Of the 36 submissions, while 14 models scored better than the baseline model (AUROC 0.627), only 2 were statistically significantly better (unadjusted p-value ≤ 0.05), though this is likely due to the relatively small size of the test set used to evaluate the models. The best model achieved an AUROC score of 0.868 (Fig. 2a).
Bootstraps (n = 1000) of the submissions for a SC1, b SC2.1, c SC2.2, and d SC2.3 ordered by submission rank. Boxes correspond to 25th, 50th, and 75th percentile, individual points are displayed beyond 1.5*IQR (interquartile range) from the edge of the box. For each sub-challenge, a baseline model using only demographic or meta-data is displayed in red as a benchmark.
For SC2.1-SC2.3, we received 35 submissions from 21 unique teams, 37 submissions from 22 unique teams, and 39 submissions from 23 unique teams, respectively (Fig. 2b–d). Due to the imbalance in severity classes, these sub-challenges were scored using the area under the precision-recall curve (AUPR). For the prediction of tremor severity (SC2.1), 16 submissions significantly outperformed the baseline model developed using only meta-data (specifically, device information, patient id, session number, site, task type, visit number, and side device was worn on) at an unadjusted p-value ≤ 0.05. The top-performing submission achieved an AUPR of 0.750 (null expectation 0.432). For the prediction of dyskinesia (SC2.2), eight submissions significantly outperformed the baseline model. The top-performing submission achieved an AUPR of 0.477 (null expectation 0.195). For the prediction of bradykinesia (SC2.3), 22 submissions significantly outperformed the baseline model. The top-performing submission achieved an AUPR of 0.950 (null expectation 0.266). While this score is impressive, it is important to note that in this case the baseline model was also highly predictive (AUPR = 0.813).
Participant approaches
The top-performing team in SC1 used a deep learning model with data augmentation to avoid overfitting (see “Methods” for details), and four of the top five models submitted to this sub-challenge employed deep learning models. In contrast, each of the winning methods for SC2.1-SC2.3 used signal processing approaches (see “Methods”). While there are differences in the data sets used for the sub-challenges (e.g., size), which could contribute to differences in which type of approach is ultimately most successful, we surveyed the landscape of approaches taken to see if there was an overall trend relating approaches and better performance. Our assessment, which included aspects of data used (e.g., outbound walk, inbound walk, and rest for the mPower data), sensor data used (e.g., accelerometer, pedometer, or gyroscope), use of pre- and post-data processing, as well as the type of method used to generate features (e.g., neural networks, statistical-, spectral- or energy-based methods), found no methods or approaches which were significantly associated with performance in any sub-challenge. This lack of statistical significance could be attributed to the large overlap in features, activities, and sensors for individual submissions in that most teams used a combination of the different methods. We also clustered submissions by the similarity of their overall approaches based on the aspects surveyed. While we found four distinct clusters for each sub-challenge, no clusters associated with better performance in either sub-challenge (Supplementary Fig. 1).
Analysis of individual features
We then turned our focus to the collection of features submitted by participants to determine which individual features were best associated with disease status (SC1) or symptom severity (SC2.1–2.3). For SC1, the 21 most associated individual features were from the two submissions of the top-performing team (which were ranked first and second among all submissions). These 21 features were also individually more informative (higher AUROC) than any of the other teams’ entire submissions (Supplementary Fig. 2B). Among the runner-up submissions, approximately half of the top-performing features were derived using signal processing techniques (36 out of 78 top features, see Supplementary Fig. 2A) with a substantial proportion specifically derived from the return phase of the walk. Interestingly, the performance of individual features in the runner-up submissions did not always correspond to the final rank of the team. For example, the best individual feature of the second-best performing team ranked 352 (out of 4546). In addition, a well-performing individual feature did not guarantee good performance of the submission (the best feature from runner-up submissions belongs to a team with a ranking 22 out of 36).
We then performed a two-dimensional manifold projection and clustered the individual features to better understand the similarity of feature spaces across teams (Supplementary Fig. 3). One of the expected observations is that the relation between features associated with the same team and the cluster membership is strongly significant (p-value~0, mean Chi-Square=8461 for t-Distributed Stochastic Neighbor Embedding (t-SNE)11 and 5402 for Multi-Dimensional Scaling (MDS)12 with k-means k > 2). This suggests most of the teams had a tendency to design similar features such that within-team distances were smaller than across-team distances (on average 26% smaller for t-SNE and 16% smaller for MDS projections). We also found that cluster membership was significantly associated with submission performance (mean p-value = 1.55E-11 for t-SNE and 1.11E-26 for MDS with k-means k > 2). In other words, features from highly performing submissions tended to cluster together. This enabled us to identify several high-performance hot-spots. For example, in Supplementary Fig. 3C a performance hot-spot is clearly identifiable and contains 51% (respectively 39%) of the features from the two best teams in SC1 (Yuanfang Guan and Marlena Duda, and ethz-dreamers), both of which employed Convolutional Neural Net (CNN) modeling. Interactive visualizations of the clusters are available online at https://ada.parkinson.lu/pdChallenge/clusters.
For each of SC2.1–2.3, we found that the best performing individual feature was not part of the respective sub-challenge winning teams’ submission, and that these best performing individual features were from submissions that have fewer features (Supplementary Fig. 4B, D, F). For SC2.2, the individual features were positively correlated with the overall submission performance (Pearson correlation = 0.12, p-value = 1.5e–05), however for SC2.1 and 2.3, a negative correlation was observed (Pearson correlation = −0.22 and −0.08 for SC2.1 and SC2.3, respectively, p-values = 1.8e−11 and 0.10). In general, individual features with modest performance, when combined, achieved better performance than feature sets with strong individual features. For SC2.1 and SC2.3 (tremor and bradykinesia), machine learning approaches showed higher performing individual features than other methods, however, signal processing-based methods showed better performing individual features in SC2.2 which, in contrast to the other two, was assessed on the resting limb (Supplementary Fig. 4A, C, E). We also attempted to improve upon the best submissions by searching among the space of submitted features for an optimal set. Attempts to optimally select features from SC2.2 using random forests or recursive feature elimination (RFE) resulted in an AUPR of 0.38 and 0.35 and placing behind the top eight and twelve individual submissions, respectively. An approach using the top principal components (PCs) of the feature space, fared slightly better, outperforming the best model in SC2.2 (AUPR = 0.504, above the top 5 feature submissions of 0.402–0.477), but failing to outperform the top models in SC2.1 and SC2.3 (AUPR = 0.674, below the top five submission scores for SC2.1; and 0.907 AUPR, within the range of the top 5 feature submissions of 0.903–0.950 for SC2.3).
Age, gender, and medication effects in mPower
Because rich covariates were available in the mPower data set, we sought to explore the prediction space created by the top submissions, in order to identify whether we could discern any patterns with respect to available covariates or identify any indication that these models could discern disease severity or medication effects (Supplementary Fig. 5). To visualize this complex space we employed topological data analysis (TDA)13 of the top SC1 submissions, to explore the grouping of subjects, firstly based on the fraction of cases with presence or absence of PD. The algorithm outputs a topological representation of the data in network form (see “Methods”) that maintains the local relationship represented within the data. Each node in the network represents a closely related group of samples (individuals) where edges connect nodes that share at least one sample. Next, we used TDA clustering to explore whether the top models showed any ability to discern symptom severity, as possibly captured by medication status (Supplementary Fig. 6). Specifically, we sought to identify whether PD patients ‘on-meds’ (right after taking medication) are more similar to controls as compared to patients who were ‘off-meds’ (right before taking medication or not taking at all). To this end, we created a topological representation for both cases, treating on-med and off-med states separately for each individual and comparing each case with the controls. Here we considered only subjects with both on-meds and off-meds sessions, to ensure the comparison was between the same population of subjects, and using only three of the top six submissions (ethz-dreamers 1, ethz-dreamers 2, and vmoroz), whose features values varied between sessions for each individual. We observed no differences in the on-meds versus off-meds TDA networks. This was consistent with the statistical analysis which showed no significant difference in the predicted PD status for patients who were on-meds versus off-meds at the time they performed their walking/balance test for any of the top models, even among patients who have previously been shown to have motor fluctuations14,15.
We then explored whether the ability of the predictive models to correctly predict PD status is influenced by factors associated with the study participants’ demographics, such as their sex, age, or the total number of walking activities they performed. We evaluated the relative performance of the top feature sets when applied to specific subsets of the test data. When comparing the predictive models’ performances in female subjects and male subjects aged 57 or older, we found that the predictive models’ were on average more accurate in classifying female subjects than male subjects with an average increase AUROC of 0.17 (paired t-test p-value = 1.4e−4) across the top 14 models (i.e., those scoring strictly better than the model using only demographic data). We note that the magnitude of the relative change is likely driven by the class balance differences between male and female subjects in the test set. In particular, a larger fraction of the female subjects aged 57 or older had a prior professional PD diagnosis than the male subjects. 80% of female subjects aged 57 or older (n = 23) had PD, and 64% of male subjects aged 57 or older (n = 66). And indeed, when compared to the demographic model, several of the top submissions are actually performing worse than the demographic model in the female subjects, while almost all are outperforming the demographic model in the male subjects (Supplementary Fig. 7). Generally, it appears that mobile sensor features are contributing more to prediction accuracy in male subjects than female subjects.
We also compared the performance of the top 14 feature sets when applied to subjects in various age groups, and found that the models performed similarly across age groups (Supplementary Fig. 7). However, in comparison to the demographic model, the top submissions perform relatively better in younger age groups (57–65) than in older age groups (65 and up), and in particular, the demographic model outperforms all of the top submissions in the highest age bracket (75 and up). This implies that the mobile features do not contribute and actually add noise in the older age brackets. Of note, the winning model by Yuanfang Guan and Marlena Duda performed well across most age and gender subgroups, but performed especially poorly in the oldest subgroup, which has the fewest samples.
To assess whether the total number of tasks performed by a subject had an impact on predictive performance, we attempted to compare subjects that had performed more tasks with those that had performed fewer. However, we found that in the mPower dataset the number of walking activities performed was predictive in itself, i.e., PD cases on average performed more tasks than the corresponding controls. We could therefore not conclusively determine whether having more data from walking activities on a subject increased the performance of the predictive models, though, related work has shown that repeatedly performed smartphone activities can capture symptom fluctuations in patients3.
Task performance across L-dopa sub-challenges
While the L-dopa data set had a small number of patients, and thus was not powered to answer questions about the models’ accuracy across demographic classes, the designed experiment allowed us to examine the predictive accuracy of the different tasks performed in the L-dopa data to understand which tasks showed the best accuracy with respect to predicting clinical severity. We scored each submission separately by task applying the same model fitting and scoring strategies used on the complete data set. For the prediction of tremor (SC2.1) and bradykinesia (SC2.3), the different tasks showed markedly different accuracy as measured by improvement in AUPR over null expectation (Supplementary Fig. 8). We observed statistically significant differences in improvement over the expected value for tremor and bradykinesia (Supplementary Tables 3–4). For tremor, activities of everyday living, such as ‘folding laundry’ and ‘organizing papers’, perform better than UPDRS-based tasks such as ‘finger-to-nose’ and ‘alternating hand movements’ (Supplementary Fig. 8, Supplementary Table 3), for which the baseline model outperformed participant submissions in almost all cases. While the ‘assembling nuts and bolts’ task showed the highest improvement over the null expectation, the baseline model also performed well, outperforming a substantial proportion of the submissions. For bradykinesia, the expected AUPR varied widely (from 0.038 for ‘pouring water’ to 0.726 for ‘alternating hand movements’). For most tasks, the participant submissions outperformed the baseline model, except in the case of the ‘alternating hand movements task’. For dyskinesia, there was no statistical difference between ‘finger-to-nose’ or ‘alternating hand movements’, but since these tasks were assessed on the resting limb, it is to be expected that this is not affected by the task being performed on the active limb.
Discussion
Given the widespread availability of wearable sensors, there is significant interest in the development of digital biomarkers and measures derived from these data with applications ranging from their use as alternative outcomes of interest in clinical trials to basic disease research1. Even given the interest and efforts toward this end, to-date there are very few examples where they have been deployed in practice beyond the exploratory outcome or feasibility study setting. This is partially due to a lack of proper validation and standard benchmarks. Through a combination of competitive and collaborative effort, we engaged computational scientists around the globe to benchmark methods for extracting digital biomarkers for the diagnosis and estimation of symptom severity of PD. With this challenge, we aimed to separate the evaluation of the methods from the data generation by using datasets that were generated but not shared with researchers and as such all participants in the challenge were naive to the data. As a resource to the community, we have shared results of the challenge, including submission rankings, methods descriptions and code, and full feature sets, as well as the specific segmentation and training/test splits of the data used in the challenge, so that other researcher can continue to improve on these methods (www.synapse.org/DigitalBiomarkerChallenge).
Participants in this challenge used an array of methods for feature extraction spanning unsupervised machine learning to hand-tuned signal processing. We did not, however, observe associations between types of methods employed and performance with the notable exception that the top two teams in the diagnostic biomarker challenge based on the mPower data (SC1) generated features using CNNs while top-performing teams in SC2.1–2.3 that used the smaller L-Dopa dataset used signal processing-derived features (though a CNN-based feature set did rank 2nd in SC2.3). The top-performing team in SC1 significantly outperformed the submissions of all remaining teams in the sub-challenge. This top-performing team was unique in its use of data augmentation, but otherwise used similar methods to the runner-up team. Consistently, deep learning has previously been successfully applied in the context of detecting Parkinsonian gait16. However, given CNNs’ relatively poorer performance in SC2, which utilized a substantially smaller dataset, we speculate that these methods may be most effective in very large datasets. This was further supported by the observation that the top SC1 model did not perform well in the oldest study subjects which correspond to the smallest age group. If the sample size is indeed a driver of the success of CNNs, this suggests that applying these methods to most digital validation datasets will not be possible as they currently tend to include dozens to hundreds of individuals in contrast to the thousands available in the SC1 data and the typical deep learning dataset17.
Traditionally, clinical biomarkers have a well-established biological or physiological interpretation (e.g., temperature, blood pressure, serum LDL) allowing a clinician to comprehend the relationship between the value of the marker and changes in phenotype or disease state. Ideally, this would be the case for digital biomarkers as well, however, machine learning models vary in their interpretability. In order to try to understand the features derived from machine learning models, we computed correlations between the CNN-derived features submitted by teams with signal processing-based features, which are often more physiologically interpretable. We were unable to find any strong linearly related signal processing analogs. Further work is necessary to try to interpret the effects being captured, though previous work has identified several interpretable features including step length, walking speed, knee angle, and vertical parameter of ground reaction force18, most of which are not directly measurable using smartphone-based applications.
Understanding the specific tasks and aspects of those activities which are most informative helps researchers to optimize symptom assessments while reducing the burden on study subjects and patients by focusing on shorter, more targeted tasks, ultimately aspiring to models for tasks of daily living instead of prescribed tasks19. To this end, given the availability of multiple tasks in SC2, we analyzed which tasks showed the best accuracy. For the tremor severity for example, the most informative tasks were not designed to distinguish PD symptoms specifically (‘pouring water’, ‘folding laundry’ and ‘organizing papers’) but mimic daily activities. However, ‘finger-to-nose’ and ‘alternating hand movements’ tasks, which are frequently used in clinical assessments, showed the lowest predictive performance, and top models did not outperform the baseline model for these tasks. For the assessment of bradykinesia, the ‘finger-to-nose’, ‘organizing paper’ and ‘alternating hand movements’ tasks showed the best model performance. However, in the case of ‘alternating-hand-movements’, the improved performance could be fully explained by the baseline model.
We believe that there are opportunities to improve the submitted models further, specifically in the sub-populations where they performed worse. For example, we observed differences in performance between males and females in the top submissions, as well as the relatively better performance in younger patients (57–65). This can be due to imbalances in the dataset such as more young participants or more data from male participants leading to better-fitting models in those populations, but could also be due to disease etiology and symptoms. We know from previous work that models can be affected by confounders20,21 and that there are gender effects of the disease22,23. If the latter is true, it is possible that different models and features might be necessary to capture different aspects of the disease as a function of age and gender. In the extreme, we might even consider personalized models15. For example, it stands to reason that the standard for normal gait differs in older people relative to younger people. Given the heterogeneity of symptom manifestation in PD, there might be many sub-populations or even idiosyncratic differences in symptom severity14. That is, the changes in disease burden as explored in SC2 might best be learned by personalized models. To help answer this question and to explore further the use of data collected in free-living conditions, we have recently launched a follow-up challenge looking at predicting personalized differences in symptom burden from data collected passively during free-living conditions.
Methods
The mPower Study
mPower7 is a longitudinal, observational iPhone-based study developed using Apple’s ResearchKit library (http://researchkit.org/) and launched in March 2015 to evaluate the feasibility of using mobile sensor-based phenotyping to track daily fluctuations in symptom severity and response to medication in PD. The study was open to all US residents, above the age of 18 who were able to download and access the study app from the Apple App Store, and who demonstrated sufficient understanding of the study aims, participant rights, and data-sharing options to pass a 5-question quiz following the consent process. Study participants participated from home and completed study activities through their mobile devices.
Once enrolled, participants were posed with a one-time survey in which they were asked to self report whether or not they had a professional diagnosis of PD, as well as demographic (Table 1) and prior-treatment information. On a monthly basis, they were asked to complete standard PD surveys (Parkinson Disease Questionnaire 824 and a subset of questions from the Movement Disorder Society Universal Parkinson Disease Rating Scale instrument25). They were also presented daily with four separate activities: ‘memory’ (a memory-based matching game), ‘tapping’ (measuring the dexterity and speed of two-finger tapping), ‘voice’ (measuring sustained phonation by recording a 10-s sustained ‘Aaaahhh’), and ‘walking’ (measuring participants’ gait and balance via the phone’s accelerometer and gyroscope). For the purpose of this challenge, we focused on the ‘walking’ test, along with the initial demographic survey data.
The walking test instructed participants to walk 20 steps in a straight line, turn around, and stand still for 30 s. In the first release of the app (version 1.0, build 7), they were also instructed to walk 20 steps back, following the 30 s standing test, however subsequent releases omitted this return walk. Participants could complete the four tasks, including the walking test, up to three times a day. Participants who self-identified as having a professional diagnosis of PD were asked to do the tasks (1) immediately before taking their medication, (2) after taking their medication (when they are feeling at their best), and (3) at some other time. Participants who self-identified as not having a professional diagnosis of PD (the controls) could complete these tasks at any time during the day, with the app suggesting that participants complete each activity three times per day.
The Levodopa Response Study
The L-dopa Response Study8,9 was an experiment with in-clinic and at-home components, designed to assess whether mobile sensors could be used to track the unwanted side-effects of prolonged treatment with L-dopa. Specifically, these side-effects, termed motor fluctuations, include dyskinesia and waning effectiveness at controlling symptoms throughout the day. In short, a total of 31 PD patients were recruited from 2 sites, Spaulding Rehabilitation Hospital (Boston, MA) (n = 19) and Mount Sinai Hospital (New York, NY) (n = 12). Patients recruited for the study came to the laboratory on Day 1 while on their usual medication schedule where they donned multiple sensors: a GENEActiv sensor on the wrist of the most affected arm, a Pebble smartwatch on the wrist of the least affected arm, and a Samsung Galaxy Mini smartphone in a fanny pack worn in front at the waist. They then performed section III of the MDS-UPDRS25. Thereafter, they performed a battery of motor tasks that included activities of daily living and items of section III of the MDS-UPDRS. This battery of tasks lasted ~20 min and was repeated 6–8 times at 30-min intervals throughout the duration of the first day. Study subjects returned 3 days later in a practically defined off-medication state (medication withheld overnight for a minimum of 12 h) and repeated the same battery of tasks, taking their medication following the first round of activities. This study also included data collection at home, between the two study visits, but these data were not used for the purposes of this challenge.
During the completion of each motor task, clinical labels of symptom severity or presence were assessed by a clinician with expertise in PD for each repetition. Limb-specific (i.e., left arm, left leg, right arm, and right leg) tremor severity score (0–4), as well as upper-limb and lower-limb presence of dyskinesia (yes or no) and bradykinesia (yes or no) were assessed. For the purposes of this challenge, we used only the GENEActiv and Pebble sensor information and upper limb clinical labels for a subset of the tasks: ‘finger-to-nose’ for 15 s (repeated twice with each arm) (ftn), ‘alternating hand movements’ for 15 s (repeated twice with each arm) (ram), ‘opening a bottle and pouring water’ three times (drnkg), ‘arranging sheets of paper in a folder’ twice (orgpa), ‘assembling nuts and bolts’ for 30 s (ntblt), and ‘folding a towel’ three times (fldng). Accelerometer data for both devices were segmented by task repetition prior to use in this challenge.
Ethics
The mPower Study was conducted remotely through an iPhone application. Participants provided consent through an interactive e-consent process, which included a quiz evaluating their understanding of the consent provided. The study and consent procedure were approved by the Western Institutional Review Board (WIRB 20181960).
Levodopa Response Study subjects were recruited and enrolled at two study sites: Spaulding Rehabilitation Hospital and Mount Sinai Hospital. All subjects signed an informed consent form. All procedures were approved by the Institutional Review Board of both study sites (Spaulding Rehabilitation Hospital IRB # 2014P000847; Mount Sinai Hospital IRB # 14-1569).
The PD Digital Biomarker Challenge
Using a collaborative modeling approach we ran a challenge to develop features that can be used to predict PD status (using data from the mPower study) and symptom severity (using data from L-dopa Response Study). The challenge was divided up into four sub-challenges, based on different phenotypes in the two different data sets. Sub-challenge 1 (SC1) focused on the extraction of mobile sensor features that distinguish between PD cases and controls using the mPower data. Sub-challenges 2.1, 2.2, and 2.3 (SC2.1-SC2.3) focused on the extraction of features that reflect symptom severity for tremor, dyskinesia, and bradykinesia, respectively, using the L-dopa data. In each case, participants were provided with a training set, containing mobile sensor data, phenotypes for the individuals represented, and all available meta-data for the data set in question. Using these data they were tasked with optimizing a set of features extracted from the mobile sensor data, which best predicted the phenotype in question. They were also provided a test set, containing only mobile sensor data, and upon challenge deadline was required to return a feature matrix for both the training and test sets. Participants were allowed a maximum of two submissions per sub-challenge, and could participate in any or all of posed sub-challenges.
For extracting features that predict PD status using the mPower data, participants were provided with up to 30-s long recordings (sampling frequency of ~100 Hz) from an accelerometer and gyroscope from 39,376 walking tasks as well as the associated 30-s recordings of standing in place, representing 660 individuals with self-reported PD and 2155 control subjects, as a training set. They were also provided with self-reported covariates, including PD diagnosis, year of diagnosis, smoking, surgical intervention, deep brain stimulation, and medication usage, as well as demographic data, including age, gender, race, education and marital status (Table 1)7. As a test data set, they were provided the same mobile sensor data from 36,664 walking/standing tasks for 614 patients with PD and 1370 controls which had not been publicly available previously, but were not provided any clinical or demographic data for these individuals. Participants were asked to develop feature extraction algorithms for the mobile sensor data which could be used to successfully distinguish patients with PD from controls, and were asked to submit features for all walking/standing activities in the training and test sets.
For the prediction of symptom presence or severity (sub-challenges 2.1–2.3), participants were provided with bilateral mobile sensor data from the L-Dopa challenge study for up to 14 repetitions of 12 separate tasks (‘drinking’ (drnkg), ‘organizing papers’ (orgpa), ‘assembling nut and bolts’ (ntblts), ‘folding laundry’ (fldng), and 2 bilateral repetitions of ‘finger-to-nose’ (ftn) and ‘(rapid) alternating hand movements’ (ram)) from 27 subjects from the L-dopa data who had sufficient quality data and completed the full protocol (Supplementary Table 2). For 19 subjects, symptom severity (tremor) or presence (dyskinesia and bradykinesia) were provided to participants as a training data set for a total of 3667 observations for tremor severity (2332, 878, 407, 38, and 12 for severity levels of 0, 1, 2, 3, and 4, respectively), 1556 observations for dyskinesia presence (1236 present), and 3016 observations for bradykinesia presence (2234 present). The data also included meta-data about the experiment such as site, device (GeneAcvtiv or Pebble), side that the device was on (left or right), day, session, and task. No demographic data was available on the study subjects at the time of the challenge. Participants were asked to provide extracted features that are predictive of each symptom for the training data, as well as 1500, 660, and 1409 observations, for tremor, dyskinesia, and bradykinesia, respectively, from the 8 test individuals for which scores were not released.
It is important to note that for each data set, the training and test sets were split by individual, that is, all tasks for a given individual fell exclusively into either the training or test set to avoid inflation of prediction accuracy from the non-independence of repeated measures on the same individual26.
The challenge website (https://www.synapse.org/DigitalBiomarkerChallenge) documents the challenge results, including links to teams’ submission write-ups and code, and links to the public repositories for the mPower and L-dopa data.
Submission scoring
For all sub-challenges, feature set submissions were evaluated by fitting an ensemble machine learning algorithm to the training observations, and predicting the test observations. The ensemble method and other metrics were chosen to process the teams’ submissions were selected to cover most major classification approaches, to avoid any bias in favor of particular modeling choices.
For SC1, we sought to minimize the undue influence from subjects who completed large numbers of walking/standing tests, by first summarizing features using the median of each feature across all observations per subject. Thus, each subject appeared only once in the training or the test set. Aggregation via the maximum showed similar results like that for the median. For each submission, elastic net (glmnet), random forests, support vector machines (SVM) with linear kernel, k-nearest neighbors, and neural net models were optimized using 50 bootstraps with AUROC as the optimization metric, and combined using a greedy ensemble in the caretEnsemble package in R. Age and sex were added as potential predictors in every submission. A subset of the provided data was used to minimize age differences between cases and controls as well as to minimize biases in study enrollment date, resulting in a training set of 48 cases and 64 controls and a testing set of 21 cases and 68 controls (Supplementary Table 1). Feature sets were ranked using the AUROC of the test predictions.
For SC2.1-2.3, the feature sets were evaluated using a soft-voting ensemble—which averages the predicted class probabilities across models—of predictive models consisting of a random forest, logistic regression with L2 regularization, and SVM (RBF kernel) as implemented in the scikit-learn Python package (0.20.0)27. The random forest consisted of 500 trees each trained on a bootstrapped sample equal in size to the training set, the logistic regression model used threefold cross-validation, and the SVM trained directly on the training set with no cross-validation and outputted probability estimates, rather than the default behavior of class scores. Other parameters were set to the default value as specified in the scikit-learn v0.20 documentation. Due to the imbalance of the class labels, we adopted the AUPR as the performance metric for the L-dopa sub-challenges. Non-linear interpolation was used to compute AUPR28. SC2.1 represents a multiclass classification problem. In order to calculate a multiclass AUPR we transformed the multiclass problem into multiple binary classification problems using the ‘one-vs-rest’ approach (where we trained a single classifier per class, with the samples of that class as positive cases and remaining samples as negative cases). For each of these binary classification problems, we computed AUPR values and combined them into a single metric by taking their weighted mean, weighted by the class distribution of the test set. SC2.2 and SC2.3 are binary classification problems, and we employed the AUPR metric directly.
For all four sub-challenges, 1000 bootstraps of the predicted labels were used to assess the confidence of the score, and to compute the p-value relative to the baseline (demographic, or meta-data) model.
Winning method sub-challenge 1: Team Yuanfang Guan and Marlena Duda
The winning method by team ‘Yuanfang Guan and Marlena Duda’ used an end-to-end deep learning architecture to directly predict PD diagnosis utilizing the rotation rate records. Separate models were nested-trained for balance and gait data, and the predictions were pooled by average when both are available. Rotation rate x, y and z were used as three channels in the network. Each record was centered and scaled by its standard deviation, then standardized to contain 4000-time points by 0-padding. Data augmentation was key to prevent overfitting to the training dataset, and was the primary difference in performance compared to the next ranking deep learning model by ‘ethz-dreamers’. The following data augmentation techniques were included to address the overfitting problem: (a) simulating people holding phones in different directions by 3D random rotation of the signal in space based on the Euler rotation formula for a standard rigid body, vertex normalized to unit = 1, (b) time-wise noise-injection (0.8–1.2) to simulate different walking speeds, and (c) magnitude augmentation to account for tremors at a higher frequency and sensor discrepancies when phones were outsourced to different manufacturers.
The network architecture was structured as eight successive pairs of convolution and max pool layers. The output of the last layer of prediction was provided as features for the present challenge. Parameters were batch size = 4, learning rate = 5 × 10−4, epoch = 50*(~half of sample size). This CNN was applied to OUTBOUND walk and REST. The networks were reseeded 10 times each. In each reseeding, half of the examples were used as training, the other half were used as validation set to call back the best mode by performance on the validation set. This resulted in multiple, highly correlated features for each task.
Winning method sub-challenge 2.1 (Tremor): Balint Armin Pataki
The creation of the winning features by team ‘Balint Armin Pataki’ was based on signal processing techniques. As PD tremor is a repetitive displacement added to the normal hand movements of a person, it can be described well in the frequency space via Fourier transformation. The main created features were the intensities of the Fourier spectrum at frequencies between 4 and 20 Hz. Observing high intensities at a given frequency suggests that there is a strong hand movement that repeats at that given frequency. In addition, hundreds of features were extracted from the accelerometer tracks via the tsfresh package29. Finally, clinical feature descriptors were created by mean-encoding and feature-binarizing the categorical clinical data provided via the scikit-learn package27. This resulted in 20 clinically derived features, 99 Fourier spectrum-based features, and 2896 features derived from tsfresh. In order to eliminate those which were irrelevant, a Random Forest classifier was applied, which selected 81 features (3 clinically derived, 6 Fourier-derived and 72 tsfresh-derived) from the ~3000 generated.
Winning method sub-challenge 2.2 (Dyskinesia): Jennifer Schaff
Data were captured using GENEActivand Pebble watch devices along several axes of motion, including the horizontal movement (side-to-side or Y-axis). Because either of these devices could be worn on the right or left wrist, an additional ‘axis’ of data was created to capture motion relative to the movement towards or away from the center of the body. This Y-axis-alt data was calculated by multiplying the Y-axis by −1 in patients that wore the device on the wrist for which the particular device (GENEActiv or Pebble) occurred less frequently. In other words, if the GENEActiv was most frequently worn on the right wrist, Y-axis measurements for left-worn measurements were multiplied by −1.
To distinguish between choreic and purpose-driven movements, summary statistics of movement along each axis per approximate second were generated, and a selection process to identify features that had the predictive potential for dyskinesia was applied. For each separately recorded task (set of patient, visit, session, and task), the absolute value of the lagged data point for each axis was calculated, and the standard deviation, variance, minimum value, maximum value, median, and sum were recorded for all variables over each approximate rolling one-second-window (51 data points). Additional features were derived by log transformation of the previously generated one-second features. To summarize across the 51 one-second values for a given task, the features were aggregated using the mean, median, sum, standard deviation, the median absolute deviation, the maximum, as well as each statistic taken over the absolute value of each observation for each variable (both original and calculated), resulting in ~1966 variables as potential features.
Random Forest model selection, as implemented within the Boruta package30 in R, was used to reduce the number of features while still retaining any variable the algorithm found to have predictive value. Any feature that was chosen by Boruta in more than 10 of 25 Boruta iterations was selected for submission, resulting in 389 variables. ‘Site’, ‘visit’, ‘session’, ‘device’, and ‘deviceSide’ as well as an indicator of medication usage were included, bringing the number of variables to 395. Features were calculated and selected for each device separately (to reduce dependency on computational resources).
Winning method sub-challenge 2.3 (Bradykinesia): Team Vision
The method by team ‘Vision’ derived features using spectral decomposition for time series and applied a hybrid logistic regression model to adjust for the imbalance in number of repetitions across different tasks. Spectral analysis was chosen for its ability to decompose each time series into periodic components and generate the spectral density of each frequency band, and determine those frequencies that appear particularly strong or important. Intuitively, the composition of frequencies of periodic components should shed light on the existence of bradykinesia, if certain ranges of frequencies stand out from the frequency of noise. Spectral decomposition was applied to the acceleration data on three axes: X (forward/backward), Y (side-to-side), Z (up/down). Each time series was first detrended using smoothing spline with a fixed tuning parameter. The tuning parameter was set to be relatively large to ensure a smooth fitted trend, so that the detrended data kept only important fluctuations. Specifically, the ‘spar’ parameter was set to 0.5 in smooth.spline function in R. It was selected by cross validation, and the error was not sensitive with spar bigger than 0.5. The tuning parameter was set the same across the tasks. The detrended time series were verified to be consistent with an autoregressive-moving-average (ARMA) model to ensure process stationarity. Following spectral decomposition, the generated features were summarized as the maximum, mean and area of estimated spectral density within five intervals of frequency bands: [0, 0.05), [0.05, 0.1), [0.1, 0.2), [0.2, 0.3), [0.3, 0.4), [0.4, 0.5]. These intervals cover the full range of spectral density. Because the importance of each feature is different for each task, features were normalized by the estimated coefficient derived by fitting separate multivariate logistic regression models for each task. The class prediction was then made based on the normalized features using logistic regression.
Analysis of methods used by participants
We surveyed challenge participants regarding approaches used. Questions in the survey pertained to the activities used (e.g., walking outbound, inbound or rest for the mPower data), the sensor data used (e.g., device motion, user acceleration, gyroscope, pedometer, etc), and the methods for extracting features from the selected data types, including pre-processing, feature generation and post-processing steps. A one-way ANOVA was conducted to determine if any use of a particular sensor, activity or approach was associated with better performance in the challenge. Significance thresholds were multiple tests corrected using a Bonferroni correction factor of 4, and no significant associations were found in any sub-challenge (p-value > 0.05 for all comparisons). We further clustered teams based on overall approach incorporating all of the dimensions surveyed. Hierarchical clustering was performed in R using the ward.d2 method and Manhattan distance. Four and three clusters were identified in SC1 and SC2, respectively. One-way ANOVA was then used to determine whether any cluster groups showed significantly different performance. No significant difference in mean scores across clusters was identified (p-value > 0.05 for all tests).
Univariate analysis of submitted features
A univariate analysis of all submitted features was performed by, on a feature-by-feature basis, fitting a generalized linear model (GLM), either logistic for SC1, SC2.2, and SC2.3 or multi-class logistic model for SC2.1, using the training samples, and predicting in the test samples. AUROC was used to measure accuracy in SC1 whereas AUPR was used in SC2.1-2.3. For SC2.1-2.3 only features from the top 10 models were assessed. Features occurring in multiple submissions (e.g., present in both submissions from the same team) were evaluated only once to avoid double counting.
Identification of optimal feature sets
In total, thousands of features were submitted for each challenge. To determine if an optimal subset of features (as defined by having a better AUPR than that achieved by individual teams) could be derived from the set of all submitted features, two different feature selection approaches were taken to identify whether choosing from all the submitted features could result in better predictive performance. These feature selection approaches were applied using only the training data to optimize the selection, and were evaluated in the test set according to the challenge methods.
First, the Boruta random forest algorithm30 was tested on the entire set of submitted features for SC2.2 (2,865), and 334 all-relevant features were selected in at least ten of 25 iterations. RFE (i.e., simple backward selection) using accuracy as the selection criteria as implemented in the caret package31 of R was then applied to the downsized feature set and selected four of the 334 features as a minimal set of features. The feature sets were then scored in the testing set per the challenge scoring algorithms, achieving AUPR of 0.38 and 0.35 for the larger and smaller sets, respectively, placing behind the top eight and twelve individual submissions for SC2.2.
A second approach applied PCA (principal component analysis) to the entire sets of features submitted for sub-challenges 2.1, 2.2, and 2.3 separately. Non-varying features were removed prior to the application of PCA. Each PC imparted only an incremental value towards the cumulative proportion of variance (CPV) explained ([maximum, 2nd, 3rd,…, median] value: [14%, 7%, 4%,…, 0.0027%], [15%, 13%, 5%,…, 0.0014%] and [15%, 7%, 6%,…, 0.00039%] for SC2.1, SC2.2, and SC2.3, respectively), suggesting wide variability in the feature space. The top 20 PCs from each sub-challenge explained 49%, 66% and 61% of the cumulative variance for SC2.1, SC2.2, and SC2.3, respectively. We then used the top PCs, which explained ~2/3 of the variation, as meta-features in each sub-challenge (50, 20, and 30 for SC2.1, SC2.2, and SC2.3, respectively), scoring against the challenge test set. These achieved an AUPR of 0.674 for SC2.1 (below the top five submission scores of 0.730–0.750), an AUPR of 0.504 AUPR for SC2.2 (above the top 5 feature submissions of 0.402–0.477) and an AUPR of 0.907 for SC2.3 (within the range of the top 5 feature submissions of 0.903–0.950).
Clustering of features
We performed a clustering analysis of all the features from SC1 using k-means and bisecting k-means with random initialization to understand the landscape of features. To map the input feature space to two dimensions for visualization while preserving the local distances, we employed two manifold projection techniques: metric MDS12 and t-Distributed Stochastic Neighbor Embedding (t-SNE)11 with various settings for perplexity, PCA dimensions, and feature standardization. The outcomes of these projections were then clustered with k-means and bisecting k-means with k = 2, 5, 10, and 20, using silhouette width32 as a cluster validity index to select the optimal number of clusters. A Kruskal–Wallis rank-sum test was used to associate cluster membership with a feature’s submission score taken as the performance of it’s associated feature set, however individual feature scores were also examined. Hot-spots were identified by binning the projected plane and smoothing the performance by a simple mean. The significance of the association between the team associated with a feature (as well as the predictive performance) with the cluster membership tends to generally increase with the number of clusters used. Clustering without PCA gives more compact and well-separated clusters and the optimal k tested by the silhouette validity index is estimated to be around 10. The clusters visualized as interactive charts are available online at https://ada.parkinson.lu/pdChallenge/clusters and the correlation networks at https://ada.parkinson.lu/pdChallenge/correlations. Visualizations of feature clusters and aggregated correlations were carried out by Ada Discovery Analytics (https://ada-discovery.github.io), performant and highly customizable data integration and analysis platform.
TDA of mPower features
To construct the topological representation, we leveraged the open source R implementation of the mapper algorithm13 (https://github.com/paultpearson/TDAmapper). As a preprocessing step, we considered only the features (median value per subject) from the six top-performing submissions in SC1, and centered and scaled each feature to obtain a z-score. We then reduced the space to two dimensions using MDS and binned the space into 100 (10 × 10) equally sized two-dimensional regions. The size of the bins was selected so that they have 15% overlap in each axis. A pairwise dissimilarity matrix based on Pearson correlation was calculated as 1-r from the original multi-dimensional space, and used to cluster the samples in each bin individually (using hierarchical single-linkage clustering). A network was generated considering each cluster as a node while forming edges between nodes that share at least one sample. Finally, we pruned the network by removing duplicate nodes and terminal nodes which only contain samples that are already accounted for (not more than once) in a paired node. We used the igraph R package (http://igraph.org/r/) to store the network data structure and Plotly’s R graphing library (https://plot.ly/r/) to render the network visualization.
Medication effects in mPower
For each submitted model to SC1, PD status was predicted for all individual walking tests in the mPower Study, regardless of reported medication status. We tested whether predicted PD status differed between patients with PD on medication (self-reported status: ‘Just after Parkinson medication (at your best)’) or off medication (self-reported status: ‘Immediately before Parkinson medication’ or ‘I don’t take Parkinson medications’) using a linear mixed model with healthCode (individual) as a random effect to account for repeated measures. We also obtained a list of individuals for whom medication status could reliably be predicted (at 5 and 10% FDR)14,15, and repeated the analysis in this subset of individuals. Results were not significant using the full set, as well as the two subsets, for any of the top 10 models, which implies that the models optimized to predict PD status could not be immediately extrapolated to predict medication status.
Demographic subgroup analysis in mPower
For each feature set, the predicted class probabilities generated by the scoring algorithm (see ‘Submission Scoring’) were used to compute AUROC within demographic subgroups by subject age group (57–60, 60–65, 65–70, and 75+) and gender (female and male). The same approach was used to assess the demographic model against which the feature sets were compared. For the purposes of this analysis, we only considered submissions that outperformed the demographic model.
Analysis of study tasks in L-dopa
For SC2.1-SC2.3, each feature set was re-fitted and rescored within each task. 1000 bootstrap iterations were performed to assess the variability of each task score for each submission. On each iteration, expected AUPR was computed based on the class distributions of the bootstrap sample. For comparison of two tasks for a given submission, a bootstrap p-value was computed as the proportion of bootstrap iterations in which AUPR(task1)-E[AUPR(task1)] > AUPR(task2)-E[AUPR(task2)]. The overall significance of the comparison between task1 and task2 was assessed via one-sided Kolmogorov–Smirnov test of the distribution, across submissions, of the p-values vs a U[0,1] distribution.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Data, predictions, feature scores, and methods descriptions used and generated in this challenge are available through Synapse (https://doi.org/10.7303/syn8717496). The mPower (https://doi.org/10.7303/syn4993293) and MJFF Levodopa Response Study (https://doi.org/10.7303/syn20681023) data are also available.
Code availability
Code used to evaluate submissions are available through https://github.com/Sage-Bionetworks/PDbiomarkerChallengeScoring. Methods descriptions and code for all available submissions is available through Synapse (https://doi.org/10.7303/syn8717496).
References
Sherman, R. E. et al. Real-world evidence - what is it and what can it tell us? N. Engl. J. Med. 375, 2293–2297 (2016).
Goldsack, J. et al. Digital endpoints library can aid clinical trials for new medicines - STAT. STAT https://www.statnews.com/2019/11/06/digital-endpoints-library-clinical-trials-drug-development/ (2019).
Zhan, A. et al. Using smartphones and machine learning to quantify Parkinson disease severity: the Mobile Parkinson Disease Score. JAMA Neurol. 75, 876–880 (2018).
Lipsmeier, F. et al. Evaluation of smartphone-based testing to generate exploratory outcome measures in a phase 1 Parkinson’s disease clinical trial. Mov. Disord. 33, 1287–1297 (2018).
Norel, R., Rice, J. J. & Stolovitzky, G. The self‐assessment trap: can we all be better than average? Mol. Syst. Biol. 7, 537 (2011).
PRO Consortium | Critical Path Institute. https://c-path.org/programs/proc/.
Bot, B. M. et al. The mPower study, Parkinson disease mobile data collected using ResearchKit. Sci. Data 3, 160011 (2016).
Daneault, JF. et al. Accelerometer data collected with a minimum set of wearable sensors from subjects with Parkinson’s disease. Sci Data 8, 48 (2021).
Vergara-Diaz, G. et al. Limb and trunk accelerometer data collected with wearable sensors from subjects with Parkinson’s disease. Sci Data 8, 47 (2021).
Ahlskog, J. E. & Muenter, M. D. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov. Disord. 16, 448–458 (2001).
van der Maaten, L. J. P. & Hinton, G. E. Visualizing high-dimensional data using t-SNE. J. Mach. Learn. Res. 9, 2579–2605 (2008).
Cox, T. F. & Cox, M. A. A. Multidimensional Scaling, 2nd edn. (CRC Press, 2000).
Singh, G., Mémoli, F. & Carlsson, G. E. Topological methods for the analysis of high dimensional data sets and 3D object recognition. Eurographics Symposium on Point-Based Graphics, https://doi.org/10.2312/SPBG/SPBG07/091-100 (2007).
Chaibub Neto, E. et al. Personalized hypothesis tests for detecting medication response in Parkinson’s Disease patients using iPhone Sensor Data. Pac. Symp. Biocomput. 21, 273–284 (2016).
Omberg, L. et al. mPower: a smartphone approach to remotely monitor Parkinson Disease and individual response to therapy. Nat. Biotech. In press.
Camps, J. et al. Deep learning for freezing of gait detection in Parkinson’s disease patients in their homes using a waist-worn inertial measurement unit. Knowl.-Based Syst. 139, 119–131 (2017).
Goodfellow, I., Bengio, Y. & Courville, A. Deep Learning. (MIT Press, 2016).
Manap, H. H., Tahir, N. M. & Yassin, A. I. M. Statistical analysis of Parkinson disease gait classification using Artificial Neural Network. IEEE Computer Society, 060–065 (Washington, DC, United States, 2011).
Powers, I. I. I. et al. Passive tracking of dyskinesia/tremor symptoms. Patent (2019).
Chaibub Neto, E., Tummalacherla, M., Mangravite, L. & Omberg, L. Causality-based tests to detect the influence of confounders on mobile health diagnostic applications: a comparison with restricted permutations. In Machine Learning for Health (ML4H) Workshop at NeurIPS 2019 - Extended Abstract. arXiv:1911.05139 (2019).
Chaibub Neto, E. et al. A permutation approach to assess confounding in machine learning applications for digital health. In Proc. 25th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining, https://doi.org/10.1145/3292500.3330903 (2019).
Haaxma, C. A. et al. Gender differences in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 78, 819–824 (2007).
Miller, I. N. & Cronin-Golomb, A. Gender differences in Parkinson’s disease: clinical characteristics and cognition. Mov. Disord. 25, 2695–2703 (2010).
Jenkinson, C., Fitzpatrick, R., Peto, V., Greenhall, R. & Hyman, N. The PDQ-8: development and validation of a short-form Parkinson’s disease questionnaire. Psychol. Health 12, 805–814 (1997).
Goetz, C. G. et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 23, 2129–2170 (2008).
Chaibub Neto, E. et al. Detecting the impact of subject characteristics on machine learning-based diagnostic applications. npj Digit. Med. 2, 99 (2019).
Pedregosa, F. et al. Scikit-learn: machine learning in python. J. Mach. Learn. Res. 12, 2825–2830 (2011).
Davis, J. & Goadrich, M. The relationship between Precision-Recall and ROC curves. In Proc. 23rd International Conference on Machine Learning - ICML ’06, 233–240 (eds Cohen, W. W. & Moore. A.) (Association for Computing Machinery, New York, NY, United States, 2006).
Christ, M., Braun, N., Neuffer, J. & Kempa-Liehr, A. W. Time Series FeatuRe Extraction on basis of Scalable Hypothesis tests (tsfresh – A Python package). Neurocomputing 307, 72–77 (2018).
Kursa, M. B. & Rudnicki, W. R., Others. Feature selection with the Boruta package. J. Stat. Softw. 36, 1–13 (2010).
Kuhn, M. Building Predictive Models in R Using the caret Package. J. Stat. Softw., Artic. 28, 1–26 (2008).
Rousseeuw, P. J. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 20, 53–65 (1987).
Acknowledgements
The Parkinson’s Disease Digital Biomarker Challenge was funded by the Robert Wood Johnson Foundation and the Michael J. Fox Foundation. Data were contributed by users of the Parkinson mPower mobile application as part of the mPower study developed by Sage Bionetworks and described in Synapse [https://doi.org/10.7303/syn4993293]. Resources and support for J.S. were provided by Elder Research, an AI and Data Science consulting agency. M.D. was supported by NIH NIGMS Bioinformatics Training Grant (5T32GM070449-12). J.F.D. was supported by a postdoctoral fellowship from the Canadian Institutes of Health Research. L.L.E. reports grants from the European Research Council ERC (677943), European Union’s Horizon 2020 research and innovation programme (675395), Academy of Finland (296801, 304995, 310561 and 314443), and Sigrid Juselius Foundation, during the conduct of the study. EG1 acknowledges the funding support by the Fonds Nationale de la Recherche (FNR) Luxembourg, through the National Centre of Excellence in Research (NCER) on Parkinson’s disease (I1R-BIC-PFN-15NCER), and as part of the grant project PD-Strat (INTER/11651464). M.K.J. was supported by Alfred Kordelin Foundation. J.J. is supported by UNSW Sydney Electronic Practice Based Research Network (ePBRN) and Translational Cancer Research Network (TCRN) programs. D.L. is supported in part by the University of Rochester CTSA award number UL1 TR002001 from the National Center for Advancing Translational Sciences of the National Institutes of Health. PS2 is supported by the Swiss National Science Foundation (SNSF) project No. 167302 within the National Research Program (NRP) 75 “Big Data”. P.S. is an affiliated PhD fellow at the Max Planck ETH Center for Learning Systems. Y.G. is supported by NIH R35GM133346, NSF#1452656, Michael J. Fox Foundation #17373, American Parkinson Disease Association AWD007950. Cohen Veterans Bioscience contributed financial support to Early Signal Foundation’s costs (U.R., C.E., and D.B.).
Author information
Authors and Affiliations
Consortia
Contributions
J.F.D., F.P., G.C., F.N.G., S.S., G.V.D., and P. Bonato designed the L-dopa Response Study and collected the data. S.K.S., P. Snyder, J.F.D., F.P., G.C., U.R., P. Banda, Y.C., E.C.N., E.R.D., F.N.G., E.R., G.V.D., D.B., P. Bonato, L.M., and L.O. designed and/or ran the challenge. S.K.S., J.S., M.D., B.A.P., M.S., P. Snyder, U.R., P.B., Z.A., A.C., L.L.E., C.E., E. Glaab, E. Goan, Y. Görmez, M.K.J., J.J., R.K., D.L., C.M.D., D.P., T.M.P., N.M.R., P. Schwab, N.S., M.S.V., Y.Z., the Parkinson’s Disease Digital Biomarker Challenge Consortium, Y.W., Y. Guan, D.B., and L.O. analyzed the data. P. Schwab, R.K., M.D., J.S., B.D., E. Goan, and D.P. lead analysis working groups.
Corresponding authors
Ethics declarations
Competing interests
J.F.D. holds equity shares of Medapplets. E.R.D. has received honoraria for speaking at American Academy of Neurology courses, American Neurological Association, and University of Michigan; received compensation for consulting services from 23andMe, Abbott, Abbvie, American Well, Biogen, Clintrex, DeciBio, Denali Therapeutics, GlaxoSmithKline, Grand Rounds, Karger, Lundbeck, MC10, MedAvante, Medical-legal services, Mednick Associates, National Institute of Neurological Disorders and Stroke, Olson Research Group, Optio, Origent Data Sciences, Inc., Prilenia, Putnam Associates, Roche, Sanofi, Shire, Sunovion Pharma, Teva, UCB and Voyager Therapeutics; research support from Abbvie, Acadia Pharmaceuticals, AMC Health, Biosensics, Burroughs Wellcome Fund, Davis Phinney Foundation, Duke University, Food and Drug Administration, GlaxoSmithKline, Greater Rochester Health Foundation, Huntington Study Group, Michael J. Fox Foundation, National Institutes of Health/National Institute of Neurological Disorders and Stroke, National Science Foundation, Nuredis Pharmaceuticals, Patient-Centered Outcomes Research Institute, Pfizer, Prana Biotechnology, Raptor Pharmaceuticals, Roche, Safra Foundation, Teva Pharmaceuticals, University of California Irvine; editorial services for Karger Publications; and ownership interests with Blackfynn (data integration company) and Grand Rounds (second opinion service). F.N.G. is currently a full time employee of Draeger Medical Systems. E.R. is an employee of Verily Life Sciences, receiving salary and equity compensation. Y.G. receives research funding from Merck KGaA, Ryss Tech and Amazon, receives personal payment from Genentech, Inc, Eli Lilly and Company, F. Hoffmann-La Roche AG, serves as chief scientist and holds equity shares at Ann Arbor Algorithms Inc. D.B. was salaried President of Early Signal Foundation at the time of this work, and is currently salaried Executive Director of Digital Health for Cohen Veterans Bioscience. P. Bonato has received grant support from the American Heart Association, the Department of Defense, the Michael J Fox Foundation, the National Institutes of Health (NIH), the National Science Foundation (NSF), and the Peabody Foundation including sub-awards on NIH and NSF SBIR grants from Barrett Technology (Newton MA), BioSensics (Watertown MA) and Veristride (Salt Lake City UT). He has also received grant support from Emerge Diagnostics (Carlsbad CA), MC10 (Lexington MA), Mitsui Chemicals (Tokyo Japan), Pfizer (New York City NY), Shimmer Research (Dublin Ireland), and SynPhNe (Singapore). He serves in an advisory role the Michael J Fox Foundation, the NIH-funded Center for Translation of Rehabilitation Engineering Advances and Technology, and the NIH-funded New England Pediatric Device Consortium. He also serves on the Scientific Advisory Boards of Hocoma AG (Zurich Switzerland), Trexo (Toronto Canada), and ABLE Human Motion (Barcelona, Spain) in an uncompensated role. Consortium authors A.A., C.A., V.C., S.H., and R.N. report that their employer, IBM Research, is the research branch of IBM Corporation, and V.C., S.H., and R.N. report that they own stock in IBM Corporation. All other authors report no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sieberts, S.K., Schaff, J., Duda, M. et al. Crowdsourcing digital health measures to predict Parkinson’s disease severity: the Parkinson’s Disease Digital Biomarker DREAM Challenge. npj Digit. Med. 4, 53 (2021). https://doi.org/10.1038/s41746-021-00414-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41746-021-00414-7
This article is cited by
-
In vivo effects of the alpha-synuclein misfolding inhibitor minzasolmin supports clinical development in Parkinson’s disease
npj Parkinson's Disease (2023)
-
Remote smartphone monitoring of Parkinson’s disease and individual response to therapy
Nature Biotechnology (2022)