Abstract
Digital medicine is an interdisciplinary field, drawing together stakeholders with expertize in engineering, manufacturing, clinical science, data science, biostatistics, regulatory science, ethics, patient advocacy, and healthcare policy, to name a few. Although this diversity is undoubtedly valuable, it can lead to confusion regarding terminology and best practices. There are many instances, as we detail in this paper, where a single term is used by different groups to mean different things, as well as cases where multiple terms are used to describe essentially the same concept. Our intent is to clarify core terminology and best practices for the evaluation of Biometric Monitoring Technologies (BioMeTs), without unnecessarily introducing new terms. We focus on the evaluation of BioMeTs as fit-for-purpose for use in clinical trials. However, our intent is for this framework to be instructional to all users of digital measurement tools, regardless of setting or intended use. We propose and describe a three-component framework intended to provide a foundational evaluation framework for BioMeTs. This framework includes (1) verification, (2) analytical validation, and (3) clinical validation. We aim for this common vocabulary to enable more effective communication and collaboration, generate a common and meaningful evidence base for BioMeTs, and improve the accessibility of the digital medicine field.
Similar content being viewed by others
Introduction
Digital medicine describes a field concerned with the use of technologies as tools for measurement and intervention in the service of human health. Digital medicine products are driven by high-quality hardware, firmware, and software that support the practice of medicine broadly, including treatment, intervention, and disease prevention, as well as health monitoring and promotion for individuals and across populations1.
Isolated silos of knowledge exist within the engineering, technology, data science, regulatory, and clinical communities that are critical to the development and appropriate deployment of digital medicine products. Currently, terminology, approaches, and evidentiary standards are not aligned across these communities, slowing the advancement of digital medicine for improved health, healthcare, and health economics. Consensus approaches are needed to evaluate the quality of digital medicine products, including their clinical utility, cybersecurity risks, user experience, and data rights and governance for ‘digital specimen’ collection2.
In this work, we refer to a specific type of digital medicine product that we call Biometric Monitoring Technologies, or BioMeTs. BioMeTs are connected digital medicine products that process data captured by mobile sensors using algorithms to generate measures of behavioral and/or physiological function. This includes novel measures and indices of characteristics for which we may not yet understand the underlying biological processes. BioMeTs, like other digital medicine products, should be characterized by a body of evidence to support their quality, safety, and effectiveness3. However, the rapid rise in the development of and demand for BioMeTs to support the practice of medicine has left in its wake a knowledge gap regarding how to develop and evaluate this body of evidence systematically4. If not addressed, there is potential for misinterpretation of data resulting in misleading clinical trials and possibly patient harm.
What are the necessary steps to determine whether a metric derived from a BioMeT is trustworthy, and by extension, whether that BioMeT is fit-for-purpose? We begin by exploring and adapting applicable concepts from other standards in related fields. Digital medicine is an interdisciplinary and rapidly evolving field. The Biomarkers, EndpointS, and other Tools (B.E.S.T) framework emphasizes that “effective, unambiguous communication is essential for efficient translation of promising scientific discoveries into approved medical products”5. Siloed and non-standardized practices will slow down innovation and impede collaboration across domains.
In this manuscript, we develop an evaluation framework for BioMeTs intended for healthcare applications. This framework includes verification, analytical validation, and clinical validation (V3). We propose definitions intended to bridge disciplinary divides and describe how these processes provide foundational evidence demonstrating the quality and clinical utility of BioMeTs as digital medicine products.
Language matters and should be used intentionally
Establishing a common language to describe evaluation standards for BioMeTs is critical to streamline trustworthy product development and regulatory oversight. In this paper, we avoid using the term “device” because we anticipate that there is a potential regulatory context for the V3 framework. We want to avoid confounding the V3 terminology with existing FDA Terms of Art (e.g., “device”). Instead, we intentionally discuss digital medicine products, and specifically BioMeTs. We refer the reader to Coravos et al for more background on regulatory considerations3. In addition, in this manuscript we use the term “algorithm” to describe a range of data manipulation processes embedded in firmware and software, including but not limited to signal processing, data compression and decompression, artificial intelligence, and machine learning.
We also avoid using the term “feasibility study.” These studies can be purposed to evaluate the feasibility of a number of performance questions and so “feasibility study” in isolation is a meaningless term. We use the term “gold standard” in quotations because it often refers to entrenched and commonly used measurement standards that are considered sub-optimal. “Gold standards” should be considered as nothing more than the best available measurement per consensus, against which the accuracy of other measurements of similar purposes may be judged6.
In this paper, we use the term “data supply chain” to describe data flow and data provenance for information generated from hardware, sensors, software, and algorithms.
Why V3?
Two terms, verification and validation, have been used for decades to describe critical components of successful quality management systems. The ISO 9000 family of quality management system standards, first published in 1987, have specific standards and definitions related to design verification and validation7. These ISO 9000 standards are generic and can be applied to any type of organization; as such, many industries have adapted these standards to their specific needs. For example, ISO 13485 specifies quality management system requirements related to design verification and validation for organizations that provide medical devices and related services8.
In the most basic sense, a BioMeT combines software and hardware for medical or health applications. The software, hardware, and regulatory parent industries have long histories of verification and validation as part of their quality management systems. Software and hardware verification and validation are guided by the IEEE Standard for System, Software, and Hardware Verification and Validation (IEEE 1012-2016), which lays out specific requirements that must be met in order to comply with the standard9. The FDA also describes verification and validation processes required for software and hardware products that are submitted for their approval10,11.
Traditional validation for software and hardware products confirms that the end product accurately measures what it claims to measure. However, BioMeT-derived measures from digital tools must also be clinically useful to a defined population. As such, we have split validation into analytical validation and clinical validation, similar to the framework used in the development of wet biomarkers and described in the BEST (Biomarkers, EndpointS, and other Tools) resource developed by the FDA-NIH Biomarkers working group5.
The three-component V3 framework is novel and intentionally combines well established practices from both software and clinical development. The definitions for V3 were derived from guidance documents, historical, and current frameworks ranging from 2002 to 2018. Each document referenced focuses on the particular audience for its associated organization(s), including system developers and suppliers, pharmaceutical industry sponsors, and regulators (Table 1). The context of the definitions provided for V3 vary greatly, highlighting that language and processes are often generated and used within disciplinary silos. Although some commonalities exist, the comparisons are confusing at best (Supplementary Table 1). These communities also lack a standard language to describe the data supply chain for information generated from the hardware, sensors, software, and algorithms.
Given (1) the historical context for the terms verification and validation in software and hardware standards, regulations, and guidances, and (2) the separated concepts of analytical and clinical validation in wet biomarkers development, this paper seeks to adapt existing terminology and evaluation frameworks for use in BioMeTs. In this new era of digital medicine, we suggest a broad interdisciplinary approach and a common lexicon containing consensus definitions across disciplines for these important terms.
Moving from current siloed practices to one universal best practice
Evaluation of BioMeTs should be a multi-step process that includes relevant expertize at each stage, as well as interdisciplinary collaboration throughout. We propose V3, a three-component framework for the evaluation of BioMeTs in digital medicine (Fig. 1):
-
1.
Verification of BioMeTs entails a systematic evaluation by hardware manufacturers. At this step, sample-level sensor outputs are evaluated. This stage occurs computationally in silico and at the bench in vitro.
-
2.
Analytical validation occurs at the intersection of engineering and clinical expertize. This step translates the evaluation procedure for BioMeTs from the bench to in vivo. Data processing algorithms that convert sample-level sensor measurements into physiological metrics are evaluated. This step is usually performed by the entity that created the algorithm, either the vendor or the clinical trial sponsor.
-
3.
Clinical validation is typically performed by a clinical trial sponsor to facilitate the development of a new medical product12. The goal of clinical validation is to demonstrate that the BioMeT acceptably identifies, measures, or predicts the clinical, biological, physical, functional state, or experience in the defined context of use (which includes the definition of the population). This step is generally performed on cohorts of patients with and without the phenotype of interest.
V3 must be conducted as part of a comprehensive BioMeT evaluation. However, although V3 processes are foundational, they are not the only evaluation steps. The concept we propose here is analogous to FDA’s Bioanalytical Method Validation Guidance for Industry13, which captures key elements necessary for successful validation of pharmacokinetic and wet laboratory biomarkers in the context of drug development clinical trials though there are some fundamental differences due to the nature of data collection tools and methods.
Clinical utility, which evaluates whether using the BioMeT will lead to improved health outcomes or provide useful information about diagnosis, treatment, management, or prevention of a disease is also necessary to determine fit-for-purpose5. To evaluate the clinical utility of a digital tool, the range of potential benefits and risks to individuals and populations must be considered, along with the relevance and usefulness of the digital product to individuals (e.g., adherence to using the technology, user experience, and battery life). Clinical utility is typically evaluated by a process of usability and user experience testing. A BioMeT may perform well under V3, but is useless if it cannot be used appropriately by the target population in the anticipated setting. However, usability, and user experience are outside of the scope of the proposed V3 framework. Other criteria, such as cost, accessibility, compatibility, burden and ease of use, failure rates, and manufacturers’ terms of use and or customer service, are also critical to determining fit-for-purpose. These are described in more detail by the Clinical Trials Transformation Initiative (CTTI)14.
How does V3 for BioMeTs fit within the current regulatory landscape?
In the United States, regulators evaluate the claim(s) a manufacturer makes for a product, rather than the product’s capabilities. In other words, a product may be categorized as a regulated “device” or “non-device” purely through a change in the manufacturer’s description of the product with no change to its functionality (e.g., no change to the hardware, firmware, or software).
The setting in which a BioMeT is used can also shift the regulatory framework. For instance, a wearable used in a clinical trial to support a drug application (e.g., to digitally collect an endpoint like heart rate) would not necessarily be considered a “device”. However, the exact same product sold in the post-market setting claiming to diagnose a condition like atrial fibrillation, would be a device under the current paradigm.
Recognizing recent shifts in the technology landscape, the US Congress signed the 21st Century Cures Act (Cures Act)15 into law on 13 December 2016, which amended the definition of “device” in the Food, Drug and Cosmetic Act to include software-based products. As a result, the FDA has been generating new guidance documents, updating policies, and considering better approaches to regulate software-driven products16. One novel approach has been to decouple the system into separate hardware and software components. For instance, the International Medical Device Regulators Forum defined ‘Software as a Medical Device (SaMD)’ as a software that performs independently of medical device hardware and that is intended to be used for medical purposes17. Importantly, this regulatory construct means that software (including algorithms), which lack a hardware component can be considered a “device” and thus, regulated by the FDA. For example, in 2018 two mobile applications that use either electrocardiogram (ECG) or photoplethymography data to generate “Irregular Rhythm Notifications” were granted De Novo clearance by the FDA18,19.
Verification
The verification process evaluates the capture and transference of a sensor-generated signal into collected data. Verification demonstrates that a sensor technology meets a set of design specifications, ensuring that (A) the sensors it contains are capturing analog data appropriately, and (B) the firmware that modifies the captured data are generating appropriate output data. In lay terms, the process of verification protects against the risk of ‘garbage in, garbage out’ when making digital measurements of behavioral or physiologic functions. BioMeTs include sensors that sample a physical construct; for example, acceleration, voltage, capacitance, or light. Verification is a bench evaluation that demonstrates that sensor technologies are capturing data with a minimum defined accuracy and precision when compared against a ground-truth reference standard, consistently over time (intra-sensor comparison) and uniformly across multiple sensors (inter-sensor comparison). The choice of reference standard depends on the physical construct captured. For example, verification of an accelerometer would involve placing the sensor on a shaking bench with known acceleration, and using these data to calculate accuracy, precision, consistency, and uniformity. In all of these processes, the evaluation criteria and thresholds should be defined prior to initiating the evaluation tests in order to determine whether the pre-specified acceptance criteria have been met.
The data supply chain
All digital measurements reported by BioMeTs are derived through a data supply chain, which includes hardware, firmware, and software components. For example, the accelerometer is a basic micro-electro-mechanical system frequently found in BioMeTs. Mechanical motion of a damped mass or cantilever in the accelerometer generates physical displacement information that can be translated through a series of data manipulations into a daily step count metric (Fig. 2; Supplementary Table 2). Each of these steps along the data supply chain has to be verified before the resulting measurement can be validated in a given population under specified conditions.
Acceleration results in physical motion of the equivalence of a spring and proof mass, which in turn results in changes of electrical properties that can be captured by electrical property sensors. Electrical signals are then converted from analog to digital signals and stored and transmitted via the microprocessor on a wristband or mobile device. Through BLE, data are then processed and compressed multiple times for transmission and storage through mobile devices or cloud storage. This figure summarizes the steps of data collection and manipulation into a daily step count metric and illustrates that “raw” data could refer to different stages of the data collection and manipulation process and have different meanings. For more details of the data types and technologies involved in each step, please refer to Supplementary Table 2. Here, two arrows are highlighted with asterisks, which signify steps in the data supply chain where the “raw data dilemma” usually occurs. What is defined and clarified as “sample-level data” are the primary and processed digital signals marked by asterisks.
The term “raw data” is often used to describe data existing in an early stage of the data supply chain. Because the data supply chains vary across BioMeTs, the definition of “raw” is often inconsistent across different technologies. Here, we define the term sample-level data as a construct that holds clear and consistent meaning across all BioMeTs. All sensors output data at the sample level (for example, a 50 Hz accelerometer signal or a 250 Hz ECG signal); these data are sometimes accessible to all users and sometimes only accessible to the sensor manufacturers. We refer to this sensor output data as d and that data are reported in the International System of Units (SI). Although signal processing methods may have been applied to this data (e.g., downsampling, filtering, interpolation, smoothing, etc.), the data are still considered “raw” because it is a direct representation of the original analog signal produced by the sensor. These are the data that must undergo verification. Unfortunately, this sample-level data are often inaccessible to third parties using those technologies. This may be owing to limitations on storage space or battery life during transmission of high frequency data or it may be due to the risk of a third party reverse-engineering proprietary algorithms developed by the BioMeT manufacturer. In these situations, only the BioMeT manufacturer can complete verification of the sample-level data.
In summary, verification occurs at the bench prior to validation of the BioMeT in human subjects. Verified sample-level data generated from the sensor technology becomes the input data for algorithms that process that data into physiologically meaningful metrics (described further in analytical validation, below). Therefore, verification serves as a critical quality control step in the data supply chain to ensure that the sample-level data meet pre-specified acceptance criteria before the data are used further.
Table 2 summarizes the process of verification.
How can we reconcile the process of verifying sensor technologies in digital medicine with approaches more familiar to other disciplines?
In both engineering and medicine, the goal of verification is to document that a specific device performs to intended specifications, but the details of the process vary with the sensor technology20. Verification itself is not defined by a fixed standard applied across all tools—rather, it is a declaration of performance with respect to a pre-specified performance goal. That performance goal is usually established by the manufacturer based on the intended use of the technology or by community standards for more common technologies, and can be precisely defined in terms that are easily testable. For example, an accelerometer’s intended performance circumstances may include the range of accelerations for which the accuracy exceeds 95% as well as the environmental and contextual conditions (e.g., temperature, humidity, battery level) for which the technology’s performance remains within that accuracy threshold. BioMeT community verification standards are typically covered by the IEC 60601 series of technical standards for the safety and essential performance of medical electrical equipment21. The series consists of collateral (IEC 60601-1-X) and particular (IEC 60601-2-X) standards. The latter define verification requirements for specific sensor signals. For instance, IEC 60601-2-26 specifies verification requirements for amplifier and signal quality properties for electroencephalography (EEG) sensors. IEC 60601-2-40 specifies similar criteria for electromyography sensors, IEC 60601-2-25 for ECG sensors, and IEC 60601-2-47 even focuses on requirements for ambulatory ECG sensors. Beyond these biopotential signals, specific standards do not exist for other commonly used sensor signals in BioMeTs (e.g., inertial, bioimpedance, and optical), leaving the definition of the verification criteria up to the manufacturer and regulatory authorities.
One challenge with establishing standard performance metrics is that performance requirements can vary by use case, and therefore the same technology performance may be sufficient for one scenario but not for another. For example, heart rate accuracy is critical for detection of atrial fibrillation in high-risk patients, but is less critical for longitudinal resting heart rate monitoring in healthy young athletes. The verification process, therefore, must include the intended use for designating appropriate thresholding criteria.
Verification serves as the initial step in a process in which data collected from further studies using the sensor technology are used to continue development of rational standards for use, uncover any unexpected sources of error, and optimize performance of BioMeTs.
Who is responsible for verification?
Verification of BioMeTs is generally performed by the manufacturer through bench-top testing. Verification tests require access to the individual hardware components and the firmware used to process the sample-level data, both of which may be proprietary; as such, in some cases it may be impractical to expect anyone other than the technology manufacturer to complete verification. Indeed, many clinical investigators utilizing the technology will not have the resources or expertize required to perform such evaluations. However, it is likely the clinical investigators who will need to define the parameters of verification that would allow a determination of whether the sensor is, indeed, fit for a particular purpose.
Technology manufacturers should provide researchers and clinical users of their tools with timely and detailed verification documentation that is easily understandable to non-technologists. This documentation should be similar to the data sheets provided for hardware components, such as individual sensors that comprise the BioMeT. The documentation of BioMeTs should include three sections: performance specifications for the integrated hardware, output data specifications, and software system tests.
Performance specifications for the integrated hardware will mimic the performance specifications for individual hardware components but the testing must be completed on the full hardware system in situ. As an example, take a simple step counting BioMeT consisting of an accelerometer sensor and associated hardware to display the current daily step count on a small screen. Verification tests for integrated hardware performance specifications could include power management (expected battery life under a variety of conditions), fatigue testing (expected lifespan of the hardware under typical and extreme use), and/or electrical conductance (expected electrical current through the BioMeT).
Output data specifications should describe the accuracy of the sample-level data produced by the BioMeT’s sensors that will be used as input to the processing algorithms to produce the processed data. These verification tests usually consist of bench-top tests. These tests are necessary even if sample-level data are passed directly to the algorithms because, at a minimum, an analog to digital conversion of the sensor data may occur within the BioMeT. In the previous example of a simple step counting BioMeT, there is only one algorithm output metric: step counts. The sample-level data that are used as an input into that algorithm are the measurements that come from the on-board accelerometer as measured in SI units. The output data specifications should detail the accuracy of the accelerometer data in each axis (e.g., ± 0.02 g) as determined through bench-top testing of the full system, not just the accelerometer sensor.
Software system tests should indicate that the entire system including software that generates the sample-level data are functioning as intended, even under unusual circumstances of use. The results of the system tests do not need to be described in exhaustive detail in the documentation; instead, a high-level description of the software system tests should be included for general knowledge. For the step counter, this could include testing to ensure that the current step count is always displayed on the screen and is incremented within 1 s of a step being detected. An unusual situation would be to test what happens when the number of steps is so great that the size of the displayed digits exceeds the size of the screen (e.g., 100,000 steps per day or more). Other system tests could include what happens when the software detects an error within the system, such as a sensor malfunction.
Overall, the verification documentation for a BioMeT should give the clinical user enough information to use the BioMeT exactly as it was designed.
What is the regulatory oversight of verification?
Regulation of verification testing in medical devices is currently overseen by the FDA in the US and the various Notified Bodies that conduct conformity assessments for CE marking in the EU22. These entities require specific verification testing before a medical device can receive clearance or approval. However, many BioMeTs are not required to go through the regulatory clearance/approval process, so independent verification standards for BioMeTs need to be developed.
There is a need for “verification standards” for BioMeTs that parallels the quality standards used to evaluate components of pharmaceuticals. In drug development, the United States Pharmacopeia23 is a non-profit organization that develops public guidelines for drug quality in collaboration with regulatory agencies, industry partners, and academia. An analogous organization for BioMeTs would be responsible for creating and updating guidelines and standards for verification testing. At present, there are multiple working groups within larger organizations that are focused on developing these verification standards for specific subsets of BioMeTs. Two examples of these working groups are the IEEE-WAMIII (Wearables and Medical IOT Interoperability & Intelligence) and the Consumer Technology Association’s Health and Fitness Technology Division. Such groups should collaborate to develop unified standards for verification that can be used by the regulatory bodies for oversight.
Table 3 describes the application of verification in practice.
Analytical validation
Analytical validation involves evaluation of a BioMeT for generating physiological- and behavioral metrics. This involves evaluation of the processed data and requires testing with human subjects24. After verified sample-level data have been generated by a BioMeT, algorithms are applied to these data in order to create behaviorally or physiologically meaningful metrics, such as estimated sleep time, oxygen saturation, heart rate variability, or gait velocity.
This process begins at the point at which verified output data (sample-level data), becomes the data input for algorithmic processing. Therefore, the first step of analytical validation requires a defined data capture protocol and a specified test subject population. For example, to develop an algorithm for gait velocity using data captured from a verified inertial measurement unit (IMU), it is necessary to specify (1) where the technology is worn (e.g., on the waist at the lumbar spine, ankle, or dominant wrist) and the orientation of the sensor, and (2) the study participant population (e.g., healthy adults aged 18–64, or patients with a diagnosis of multiple sclerosis aged 5–18)25,26. In this example, the analytical validation consists of evaluating the performance of the gait velocity algorithm on verified IMU data captured in accordance with the specific study protocol and in the particular study population of healthy adults aged 18–64.
During the process of analytical validation, the metric produced by the algorithm must be evaluated against an appropriate reference standard. Sleep onset/wake, for example, should be validated against polysomnography; oxygen saturation against arterial blood samples; heart rate variability against electrocardiography; and biomechanics such as gait dynamics against motion capture systems. It is important to remember that there can be multiple reference standards for a single metric, and not all reference standards are based on sensors. For example, a commonly used reference standard for respiratory rate is a manual measurement: a nurse observes and counts a study participant’s chest raises over a defined period of time. Manual reference standards are necessary when it is infeasible or impractical to use a sensor-based standard; step counts, for example, are typically validated using manual step counting rather than an instrumented walkway or instrumented shoes because it is more practical to have a human observer manually count the subject’s steps during a long walk test. In general, however, manual measurements are not the best choice for reference standards as they are the most prone to user error; they should only be used when absolutely necessary and no other reference standards are suitable and/or feasible.
It would be counterproductive to recommend a single threshold of accuracy for analytical validation of a BioMeT metric versus a reference standard as not all reference standards are of equal quality. First, not all reference standards are completely objective. For example, polysomnography signals are collected via sensors but may be manually scored by a trained technologist to generate sleep variables. Second, ostensibly objective reference standards like optical motion capture systems may have substantial operator bias that increases the variability of the final measurements27. Finally, in some cases a “gold standard” reference standard may not be clearly defined. For example, Godfrey et al. noted that the validation process for metrics produced by a gait algorithm based on body worn inertial sensors compared with a traditional laboratory reference standard, an instrumented pressure sensor gait mat, revealed poor agreement for variability and asymmetry estimates of left/right step data. In this case, a gait mat is a poor choice of reference standard to evaluate body worn sensors due to fundamental differences in measurement methods between the pressure and inertial sensor modalities28. Therefore, we recommend caution in the choice of reference standards for analytical validation studies. Most importantly, it is critical to understand how the selected reference standard measures and interprets the desired metric in order to undertake appropriate analytical validation procedures.
Best practices should be followed when choosing a reference standard for analytical validation of a BioMeT. The most rigorous and quantitative reference standards should be agreed upon and documented by guidance documents and consensus statements from governance and professional organizations. These are the reference standards that should be selected in order to avoid poor methodological approaches. Low-quality reference standards have the potential to introduce error as they may only produce an estimate of the desired metric. For example, a sleep diary contains the subject’s recollection of their sleep onset/wake time, which might vary considerably from the actual sleep onset/wake. Similarly, the process of back-validation, where analytical validation of a next generation BioMeT is evaluated against the previous generation, will also introduce error that can quickly compound if this process is repeated over multiple generations.
Table 4 summarizes the process of analytical validation.
How can we reconcile analytical validation of BioMeT-generated measures in digital medicine with more familiar approaches from other disciplines?
BioMeTs come in a wide variety of form factors and levels of complexity. Despite this variation, the goals and challenges of generating evidence of analytical validity are common across many tools and are similar to those of non-digital tools. For example, both assessing the analytical validity of heart rate variability (HRV) from a commercial chest strap and gait velocity from a wrist-worn accelerometer require the use of reference standards, testing protocols, and statistical analyses that are widely accepted by subject matter experts. These elements have been a part of analytical validation within engineering and health-related disciplines for many years. However, questions of their relevance to BioMeTs of ever-increasing novelty can arise, particularly when the reference standards, testing protocols, and statistical analyses are poorly defined, non- intuitive, or are not disclosed at all.
In some instances, a BioMeT may be attempting to replace a less-robust clinical measurement tool that provides only measurement estimates (i.e., patient diaries). When it is not possible to robustly establish analytical validation due to the novelty of the data type generated from a BioMeT (i.e., no reference standard exists), then the need for evidence of clinical validity and utility increases. In contrast, the primary element required to demonstrate clinical validity (discussed below) is a reproducible association with a clinical outcome of interest. Methodological approaches to establishing associations are diverse and the most appropriate methods are dependent on the target population and context of clinical care.
Who is responsible for analytical validation?
Analytical validation focuses on the performance of the algorithm and its ability to measure, detect, or predict the presence or absence of a phenotype or health state and must involve assessment of the BioMeT on human participants. As such, the entity that is developing the algorithm is responsible for analytical validation. Ideally, analytical validation would benefit from collaboration between the engineering team responsible for developing the sensor technology, data scientists/analysts/statisticians, physiologists or behavioral scientists, and the clinical teams responsible for testing in human participants from which the data are captured and the algorithm is derived. These multi-disciplinary teams might all sit within a single organization or may be split between a technology manufacturer and an analytics company, academic organization, and/or medical product manufacturer.
Commercial technology manufacturers often focus on developing generic algorithms with broad applications to a wide variety of subject populations in order to market their products to the widest possible consumer base. These algorithms (step count, walking speed, heart rate and heart rate variability, falls, sleep, muscle activation, etc.) could be applied to subjects with a variety of health conditions and under a variety of circumstances. However, commercial technology manufacturers may only conduct analytical validation for their algorithms using a small cohort of healthy subjects in a controlled laboratory setting. The manufacturer may or may not document the results of these studies in order to demonstrate the analytical validation of all the algorithms in their product. Sponsors of new medical products (drugs, biologics, or devices) choosing to use commercial technology will typically need to conduct their own analytical (and then clinical) validation.
When sponsors of new medical products (drugs, biologics, or devices) want to use BioMeTs to assess safety or efficacy of a new medical product for regulatory approval, they necessarily focus on developing specific algorithms with narrow applications that are targeted to their exact patient population of interest (e.g., Parkinson’s disease, multiple sclerosis, Duchenne’s muscular dystrophy). Through their clinical trial populations, sponsors generally have access to large data sets of patients with the specific health condition of interest from which to develop their algorithms. The trial sponsors may include a BioMeT prospectively as an exploratory measure in a clinical trial (both early and late stage) and use the collected data to develop the algorithm. There may be no available reference standards for these targeted algorithms; as a result, the sponsor may use other data collected during the clinical trial as the surrogate reference standards for the algorithms.
The sponsor should thoroughly document the analytical validation of the algorithms and is required to submit these results to regulatory bodies such as FDA or EMA. However, owing to the sensitivity of data collected during a clinical trial, these results may never be published or may be published years after the clinical trial has concluded. To demonstrate the efficacy of the BioMeT, we recommend that sponsors publish the results of analytical validation as soon as possible.
Table 5 describes the application of analytical validation in practice.
Clinical validation
Clinical validation is the process that evaluates whether the BioMeT acceptably identifies, measures, or predicts a meaningful clinical, biological, physical, functional state, or experience in the specified context of use. An understanding of what level of accuracy, precision, and reliability is necessary for a tool to be useful in a specific clinical research setting is necessary to meaningfully interpret results.
Clinical validation is intended to take a measurement that has undergone verification and analytical validation steps and evaluate whether it can answer a specific clinical question. This may involve assessment or prognosis of a certain clinical condition. Clinical validation should always be tailored to a specific context of use. The goal of clinical validation is to evaluate the association between a BioMeT-derived measurement and a clinical condition. The process of clinical validation also ensures the absence of systemic biases and can uncover BioMeT limitations such as an improper dynamic range to address a particular question. For example, a clinical validation could be determined in a study assessing the relationship between ambulatory BP monitoring and all-cause and cardiovascular mortality29.
Developing a standardized framework for clinical validation is challenging because of the highly variable nature of questions asked of clinical validation studies. However, we can adapt solutions from the FDA Guidance on patient reported outcomes30 or the CTTI recommendations and resources for novel endpoint development31. Some of the concepts such as defining meaningful change to interpret treatment response and ability to detect clinically meaningful change could be leveraged more extensively for the purposes of clinical validation for BioMeTs.
Clinical experts, regulators, and psychometricians who are experienced with the development of clinical measurement tools are intimately familiar with the process of clinical validation. The work that these experts do, does not change when the tool is digital.
Table 6 summarizes the process of clinical validation.
How can we reconcile clinical validation of sensor-generated measures in digital medicine with more familiar approaches from other disciplines?
Clinical validation is a process that is largely unique to the development of tests, tools, or measurements either as medical products themselves, or to support safety and/or efficacy claims during the development of new medical products, or new applications of existing medical products. Technology manufacturers who are not yet experienced in the clinical field may be unfamiliar with this final step in the development of a BioMeT. Equally, clinical experts with significant experience developing traditional clinical tests, tools, and measurement instruments may not realize that this process does not vary when developing and evaluating a BioMeT.
Who is responsible for clinical validation?
Clinical validation is conducted by clinical teams planning to use, or promote the use of, the BioMeT in a certain patient population for a specific purpose. In practice, sponsors of new medical products (drugs, biologics, or devices) or clinical researchers will be the primary entities conducting clinical validation. If the digital tool is being used to support a labeling claim in the development of a new medical product, or a new application of an existing medical product, then the sponsor of the necessary clinical trials will be required to conduct clinical validation of any BioMeTs they use to make labeling claims.
In circumstances where the sponsor has completed analytical validation of an algorithm for a specific and narrow patient population, it may be possible to reuse some of the patient data that informed analytical validation to complete clinical validation. Clinical trials (both early and late stage) generate large data sets of patient health data that have traditionally been used to demonstrate clinical validity of biomarkers or surrogate endpoints5. This same process still applies when evaluating BioMeTs. We recommend using caution to avoid overestimating the utility of a digital endpoint if the same data set is used for both analytical and clinical validation. Documentation of clinical validation for BioMeTs should follow the same processes and requirements of clinical validation of traditional tests, tools, and measurement instruments32.
Table 7 describes the application of clinical validation in practice.
What is the regulatory oversight of the analytical and clinical validation processes?
The pathways for regulatory oversight of the validation processes will vary with the claims that the manufacturer of the BioMeT makes. For BioMeTs on regulatory pathways that require clearance or approval as a medical device, the centers within regulatory bodies responsible for these devices have regulatory oversight. These pathways are described in detail in Digital Medicine: A Primer on Measurement3.
For BioMeTs being used to support safety and efficacy claims of other medical products, there are a number of different options. In the United States, there is a pathway to “qualify” a digital tool outside of an individual drug development program32. Other pathways are specific to the medical product of interest. Decisions about the best approach to developing and/or a BioMeT in clinical trials and the preferred approaches for analytical validation should be made with input from regulatory agencies. CTTI has developed a quick reference guide to engage with the FDA for these conversations33.
Real-world examples of V3 processes
Table 8 describes the application of V3 processes for five use cases, including both commercial and medical BioMeTs.
The V3 framework in practice
There are a number of considerations that transcend the processes of verification and analytical validation, and clinical validation in the development of BioMeTs.
Do these processes replace existing GxP processes?
No. Good ‘x’ practices (or GxP) are guidelines that apply to a particular field. For example, ‘x’ may be manufacturing (GMP) or laboratory (GLP). Good practice guidelines apply to products in regulated fields (e.g., pharmaceuticals and medical devices) and are intended to ensure that these products are safe and meet their intended use by complying with strict quality standards throughout the entire process of production. V3 processes should be applied to all BioMeTs used in digital medicine. Digital tools that are also cleared or approved as medical devices must also comply with applicable GxP guidelines.
Emphasizing the importance of a study protocol during V3 evaluation
It is important to develop clear study protocols and reports prior to embarking on V3 exercises. For verification, documentation should stipulate the requirements/acceptance criteria, testing steps, procedures, timelines, and documentation of the experimental results with appropriate conclusions. Both analytical validation and clinical validation processes are subject to regulations applicable to human experimentation. Clinical study protocols are required with an approval of IRB/EC and regulatory agencies, as applicable.
For all V3 processes, keeping appropriate test/study protocols and reporting the results is critical as it serves multiple purposes: defining the objectives of the experiment, aligning all stakeholders involved, complying with applicable regulations, and providing tools for determining compliance. In addition, protocols and study reports are key tools for documenting scientific evidence needed to draw inferences on whether a technology is fit-for-purpose for the intended use and context of use.
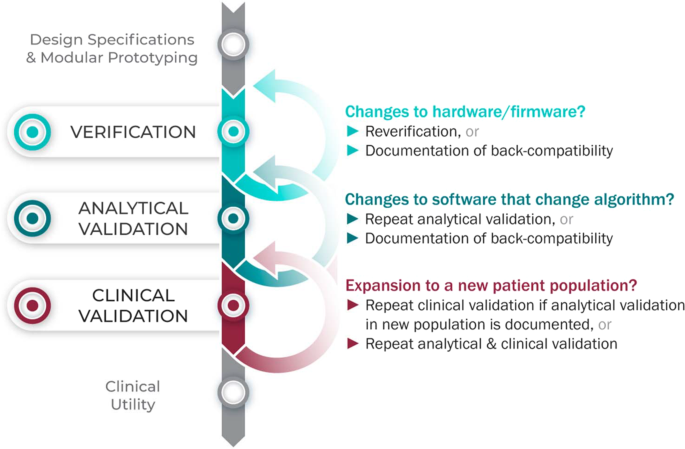
Considering upgrades to firmware and/or software
The requirements for V3 are determined by the intended use of the BioMeT. Therefore, if the hardware or software are changed, new verification and/or analytical validation studies are needed to provide updated documentation for the end user (e.g., the study sponsor using the BioMeT as a drug development tool). Fortunately, changes in hardware and firmware often have no negative effects on the sample-level data, but the manufacturer still needs to demonstrate that this is true and also whether there is a “backwards compatibility” with earlier models. This is important because if an engineering improvement in BioMeT firmware or hardware makes the new data incompatible with data collected from earlier versions, this “improvement” could be disastrous for longitudinal studies and meta analyses.
Software updates that include changes to the algorithm processing the sample-level data require analytical validation to be repeated. However, if the hardware and firmware are unchanged, it is not necessary to repeat verification and analytical validation can be conducted using pre-existing sample-level data.
There can be misperceptions of the implications of firmware and software updates, such as whether or not those trigger new reviews from regulators like the FDA. For instance, software manufacturers are able—and encouraged by the FDA—to patch known security vulnerabilities34. Notably, software manufacturers, and not the FDA, are responsible for 640 validation of software changes after the patch has been deployed34.
Extending BioMeTs to new populations
If the BioMeT itself has not changed, it is not necessary to repeat existing verification studies. However, whether existing validation data can be generalized to a different patient population or clinical setting is also a matter for scientific judgment and may require additional analytical validation and clinical validation studies. For example, consider an algorithm that processes data from a hip-worn accelerometer to generate the number of steps per day that was originally developed using data collected from healthy college athletes. There may be published data demonstrating that the algorithm performs well when tested on similar populations, such as people who are slightly older or those who are generally fit and active. However, it is unlikely, that the algorithm will generate an accurate step count if applied to a person suffering from peripheral neuropathy or a gait disorder. Thus, it would be incorrect to assume that just because analytical validation testing has demonstrated good performance in one scenario that the algorithm is then validated for use in all scenarios.
Extending V3 concepts to multimodal and composite digital measures
V3 processes extend to multimodal data and composite digital measures. Multimodal describes data captured from two or more unique measurement methods. For example, a combination of accelerometer and gyroscope data can be used to detect falls and sit-to-stand transitions35,36. Digital tools relying on multimodal data should have evidence of verification available for each sensor, and evidence of analytical validation and clinical validation for the measure itself. Composite digital measures combine several individual measures, often derived from different sensors, to reach a single interpretive readout. For example, combining digital assessments of heart rate, sleep and heart rate variability can render a composite measure of depression37. Another example may combine accelerometer, GPS, keyboard and voice data from a smartphone to give a composite measure of cognition38. In these cases, verification of all contributing sensors is required along with validation of both the individual measures and the combined composite measure.
How much validation is “enough”?
It can be difficult to decide whether an analytical validation study has achieved its goal of determining that an algorithm correctly captures the behavioral or physiological measure of interest. If there is a clear and objective reference standard, then a numerical accuracy threshold can be set a priori, and the algorithm can be said to be sufficiently well validated if the results of the testing meet or exceed the threshold. A numerical accuracy threshold should be chosen based on the expected accuracy of the reference standard combined with a literature review of relevant research and comparable validation studies that indicate what would be clinically meaningful accuracy. For example, heart rate has a clear reference standard (multi-lead ECG) and there are many published analytic validation studies describing the accuracy of various heart rate measurement devices39.
When evaluating a novel metric where there is no clear reference standard, analytical validation becomes a more challenging task. In such cases, the first step is to determine what level of accuracy is necessary to be clinically meaningful in the expected user population. This can be determined by a literature review of previously published research combined with consultations of key opinion leaders in the field. Once an approximate accuracy threshold has been established, the best available reference standard should be chosen. The reference standard is often the measurement method used in clinical practice, and should be chosen based on the literature and in consultation with key opinion leaders. Then the analytical validation study can be completed. It is noteworthy that the absence of a clear reference standard necessarily requires the integration of elements of analytical and clinical validation to appropriately evaluate the measure. An example of this type of study is the measurement of tremor in patients with Parkinson’s disease. Tremor is usually assessed by visual observation of the patient, which is not a clear reference standard. In one study, a BioMeT’s measurement of Percent of Time that Tremor is Present in Parkinson’s patients was assessed against visual observation to generate an accuracy score40.
In general, it is not possible to set a blanket threshold for all types of statistical assessments of clinical validation, as these will differ depending on the clinical measurement, patient population, and context of use. For example, a BioMeT that is highly sensitive to detecting a disease may be valuable for the purposes of screening owing to the low false-negative rate, whereas a BioMeT that is highly specific may be of value for the purpose of diagnosis owing to the low false-positive rate. Second, determining that the endpoint generated by the BioMeT is clinically valid and of importance to understanding the functional status or quality of life of the target population is critical. This process relies on examining the totality of evidence related to the endpoint in question, and using that information to make a scientific judgment as to whether the endpoint is an appropriate measurement or diagnostic marker. For clinical validation, the best practice would be to publish all available testing and results (including the protocols), which will allow future users to choose the most appropriate BioMeT for their specific purpose (fit for purpose).
Figure 3 summarizes the application of the V3 process in the real world.
Statistical considerations in V3
Error can stem from a wide array of sources when employing BioMeTs. The development and implementation of a robust V3 protocol and subsequent BioMeT deployment and use in accordance with that V3 protocol will minimize error resulting from differences between expected and actual accuracy as well as intended and actual use. There are a wide range of statistical analyses used to evaluate BioMeTs for their coherence with reference standards and their clinical power, which is beyond the scope of this paper. Provision of raw data, whenever possible, helps to address transparency and independent evaluation of technologies by allowing independent investigation of, for example, data variance and its impact on BioMeT reliability. In addition, it is important to consider the limits of agreement if using different devices to quantify the same biomarker at different timepoints or in different cohorts.
Future directions
Digital medicine is an interdisciplinary field, drawing together stakeholders with expertize in engineering, manufacturing, clinical science, data science, biostatistics, regulatory science, ethics, patient advocacy, and healthcare policy, to name a few. Although this diversity is undoubtedly valuable, it can lead to confusion regarding terminology and best practices in this nascent field. There are many instances, as we detail in this paper, where a single term is used by different groups to mean different things, as well as cases where multiple terms are used to describe what is essentially the same concept. Our intent is to clarify the core terminology and best practices for the evaluation of BioMeTs for use in clinical trials of new medical products, without unnecessarily introducing new terms. We aim for this common vocabulary to enable more effective communication and collaboration while improving the accessibility of the field to new adopters.
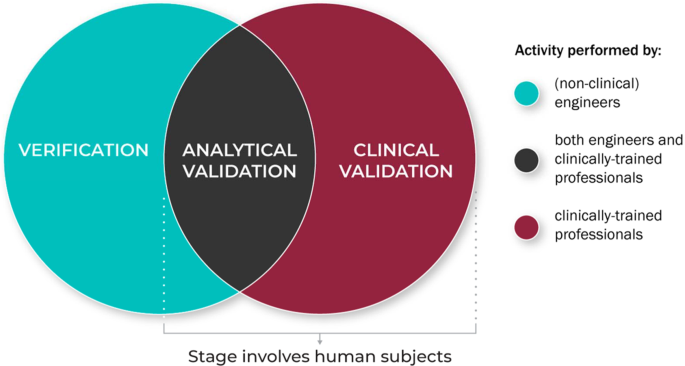
Figure 4 summarizes the role of the different disciplinary experts in the V3 process.
V3 processes for traditional medical devices are generally well established but BioMeTs introduce new considerations41. For instance, SaMDs do not rely on specific hardware or sensors. The process of verification enables the use of SaMDs on verified data from any suitable sensor technology. In addition, some vendors sell “black box” algorithms or combined sensor/algorithm pairings. Establishing clear definitions and evidentiary expectations for the V3 processes will support collaborators seeking to evaluate the output of a “black box” sensor technology and/or measurement tool. Although the focus of this manuscript is on the use of BioMeTs in regulated trials of new medical products, our intent is for this framework to be instructional to all users of digital measurement tools, regardless of setting or intended use. Informing treatment decisions or care management based on a digital measure should not be subject to different scrutiny. Our goal in advancing this unifying V3 evaluation framework is to standardize the way high-quality digital measures of health are developed and implemented broadly. Evidence to support a determination of ‘fit-for-purpose’ and build trust in a digital measure should be uniform. A lack of V3 evaluation will have severe consequences (see Table 9 for illustrative examples) if algorithms fail to run according to predetermined specifications or if BioMeTs fail to perform according to their intended purpose.
Adopting streamlined methods for transparent reporting of V3 methodologies could lead to more ubiquitous deployment of low-cost technologies to better assess and monitor people outside of the clinic setting. This in turn can help healthcare professionals better diagnose, treat, and manage their patients, whereas promoting individualized approaches to medicine. Transparency will overcome “black box” technology development and evaluation approaches, ensuring that BioMeTs are used appropriately with the robust capture of data regardless of environment and context.
The proposed V3 process for BioMeTs describes an evidence base to drive the appropriate adoption of fit-for-purpose digital measurement technologies. In this document, we propose this three-pronged framework using historic and current contexts to define the key terms in this process. As a next step, we strongly encourage a re-initiation of the FDA B.E.S.T. working group to consider these definitions, refine them, and add them to the working compendium BEST framework42. We also encourage groups like the IEEE to consider these ontologies and provide feedback and guidance on the next steps required to adopt a common language and model for digital tools. We also recognize that technological developments will move faster than any regulatory or standards body can keep up with, so we encourage the practitioners in the digital era of medicine, including data scientists, engineers, clinicians and more, to continue to build upon this work. Professional societies like The Digital Medicine Society (DiMe) aim to become a collaborative hub for innovation in this area. Our hope is that the V3 framework and definitions continue to evolve to reflect the technologies that they serve. Our team will aim for annual updates to the framework as it exists herein. Once a common BioMeT evaluation paradigm is agreed upon, we will be able to develop technologies deserving of the trust we place in them (Boxes 1–3).
References
Goldsack, J. Laying the Foundation: Defining Digital Medicine. Medium (2019). Available at: https://medium.com/digital-medicine-society-dime/laying-the-foundation-defining-digital-medicine-49ab7b6ab6ef. (Accessed 18 Sept 2019).
Perakslis, E. & Coravos, A. Is health-care data the new blood? Lancet Digital Health 1, e8–e9 (2019).
Coravos, A. et al. Digital medicine: a primer on measurement. Digit Biomark. 3, 31–71 (2019).
Dunn, J., Runge, R. & Snyder, M. Wearables and the medical revolution. Per. Med. 15, 429–448 (2018).
FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource. (Food and Drug Administration (US), 2016).
Versi, E. ‘Gold standard’ is an appropriate term. BMJ 305, 187 (1992).
14:00-17:00. ISO 9001:2015. ISO Available at: http://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/06/20/62085.html. (Accessed 18 Sept 2019).
International Organization for Standardization & International Electrotechnical Commission. ISO 13485:2016, Medical devices — Quality management systems — Requirements for regulatory purposes. (2016).
IEEE Computer Society. IEEE Standard for System, Software, and Hardware Verification and Validation. IEEE Std 1012-2016 (Revision of IEEE Std 1012-2012/ Incorporates IEEE Std 1012-2016/Cor1-2017) 1–260 (2017). https://doi.org/10.1109/IEEESTD.2017.8055462.
U.S. Department Of Health and Human Services, U.S. Food and Drug Administration, Center for Devices and Radiological Health & Center for Biologics Evaluation and Research. General Principles of Software Validation; Final Guidance for Industry and FDA Staff, 47 (2002).
U.S. Food and Drug Administration. CFR - Code of Federal Regulations Title 21. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=820.30. (Accessed 18 Sept 2019).
Center for Drug Evaluation and Research. Drug Development Tool Qualification Programs. FDA (2019). Available at: http://www.fda.gov/drugs/development-approval-process-drugs/drug-development-tool-qualification-programs. (Accessed 18 Sept 2019).
U.S. Food and Drug Administration. Bioanalytical Method Validation Guidance for Industry. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry. (Accessed 7 Mar 2020).
Clinical Trials Transformation Initiative. Framework of Specifications to Consider During Mobile Technology Selection (2018).
H.R. 34, 114th Congress. 21st Century Cures Act (2016).
U.S. Food and Drug Administration. Digital Health Innovation Action Plan. (2017). https://www.fda.gov/media/106331/download.
IMDRF SaMD Working Group. Software as a Medical Device (SaMD): Key definitions (2017).
Krueger, A. C. Regulation of photoplethysmograph analysis software for over-the-counter use. U.S. Food & Drug Administration (2018).
Krueger, A. C. Regulation of electrocardiograph software for over-the-counter use. U.S. Food & Drug Administration (2018).
Bignardi, G. E. Validation and verification of automated urine particle analysers. J. Clin. Pathol. 70, 94–101 (2017).
International Electrotechnical Commission. Available at: https://www.iec.ch/. (Accessed 18 Sept 2019).
Margaine, C. The Notified Body’s Role in Medical Device Certification. Available at: https://lne-america.com/certification/ce-marking-gain-market-access-to-europe/notified-body. (Accessed 18 Sept 2019).
USP (The United States Pharmacopeial Convention). Available at: https://www.usp.org/. (Accessed 18 Sept 2019).
Witt, D. R., Kellogg, R. A., Snyder, M. P. & Dunn, J. Windows into human health through wearables data analytics. Curr. Opin. Biomed. Eng. 9, 28–46 (2019).
McCamley, J., Donati, M., Grimpampi, E. & Mazzà, C. An enhanced estimate of initial contact and final contact instants of time using lower trunk inertial sensor data. Gait Posture 36, 316–318 (2012).
Trojaniello, D., Cereatti, A. & Della Croce, U. Accuracy, sensitivity and robustness of five different methods for the estimation of gait temporal parameters using a single inertial sensor mounted on the lower trunk. Gait Posture 40, 487–492 (2014).
Hutchinson, L. et al. Operator bias errors are reduced using standing marker alignment device for repeated visit studies. J. Biomech. Eng. 140, 041001 (2018).
Godfrey, A., Del Din, S., Barry, G., Mathers, J. C. & Rochester, L. Instrumenting gait with an accelerometer: a system and algorithm examination. Med. Eng. Phys. 37, 400–407 (2015).
Banegas, J. R. et al. Relationship between clinic and ambulatory blood-pressure measurements and mortality. N. Engl. J. Med. 378, 1509–1520 (2018).
U.S. Department of Health and Human Services, U.S. Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER) & Center for Devices and Radiological Health (CDRH). Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. (2009).
Clinical Trials Transformation Initiative. CTTI Recommendations: Developing Novel Endpoints Generated by Mobile Technology for Use in Clinical Trials. (2017).
U.S. Department of Health and Human Services, U.S. Food and Drug Administration, Center for Drug Evaluation and Research (CDER) & Center for Biologics Evaluation and Research (CBER). Biomarker Qualification: Evidentiary Framework Guidance for Industry and FDA Staff. (2018).
Clinical Trials Transformation Initiative. Quick Reference Guide to Processings for Interacting with the US Food and Drug Administration (FDA) regarding Novel Endpoint Development. (2017).
U.S. Food and Drug Administration. FDA Fact Sheet: The FDA’S Role in Medical Device Cybersecurity, Dispelling Myths and Understanding Facts.
Huynh, Q. T., Nguyen, U. D., Irazabal, L. B., Ghassemian, N. & Tran, B. Q. Optimization of an accelerometer and gyroscope-based fall detection algorithm. J. Sens. (2015). https://doi.org/10.1155/2015/452078.
Pham, M. H. et al. Validation of a lower back “wearable”-based sit-to-stand and stand-to-sit algorithm for patients with parkinson’s disease and older adults in a home-like environment. Front. Neurol. 9, 652 (2018).
Kovalchick, C. et al. Can composite digital monitoring biomarkers come of age? A framework for utilization. J. Clin. Transl. Sci. 1, 373–380 (2017).
Insel, T. R. Digital phenotyping: technology for a new science of behavior. JAMA 318, 1215–1216 (2017).
Wang, R. et al. Accuracy of wrist-worn heart rate monitors. JAMA Cardiol. 2, 104 (2017).
Braybrook, M. et al. An ambulatory tremor Score for parkinson’s disease. J. Parkinsons Dis. 6, 723–731 (2016).
Panescu, D. Medical device development. in 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society 5591–5594 (2009). https://doi.org/10.1109/IEMBS.2009.5333490.
Commissioner, O. of the. FDA in brief: FDA seeks public feedback on biomarker and study endpoint glossary. FDA (2019).
IEEE Standard for System, Software, and Hardware Verification and Validation. IEEE Std 1012-2016 (Revision of IEEE Std 1012-2012/ Incorporates IEEE Std 1012-2016/Cor1-2017) 1–260 (2017). https://doi.org/10.1109/IEEESTD.2017.8055462.
National Academies of Sciences, Engineering, and Medicine. An Evidence Framework for Genetic Testing. (The National Academies Press, 2017). https://doi.org/10.17226/24632.
Giles, D., Draper, N. & Neil, W. Validity of the Polar V800 heart rate monitor to measure RR intervals at rest. Eur. J. Appl. Physiol. 116, 563–571 (2016).
Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task force of the European society of cardiology and the north american society of pacing and electrophysiology. Circulation 93, 1043–1065 (1996).
Hernando, D., Garatachea, N., Almeida, R., Casajús, J. A. & Bailón, R. Validation of heart rate monitor polar rs800 for heart rate variability analysis during exercise. J. Strength Cond. Res. 32, 716 (2018).
Frasch, M. G. et al. Can a heart rate variability biomarker identify the presence of autism spectrum disorder in eight year old children? arXiv:1808.08306 [q-bio] (2018).
Karpman, C., LeBrasseur, N. K., DePew, Z. S., Novotny, P. J. & Benzo, R. P. Measuring gait speed in the out-patient clinic: methodology and feasibility. Respir. Care 59, 531–537 (2014).
Fortune, E., Lugade, V., Morrow, M. & Kaufman, K. Validity of using tri-axial accelerometers to measure human movement – Part II: step counts at a wide range of gait velocities. Med. Eng. Phys. 36, 659–669 (2014).
König, A. et al. Objective measurement of gait parameters in healthy and cognitively impaired elderly using the dual-task paradigm. Aging Clin. Exp. Res 29, 1181–1189 (2017).
U.S. Department of Health and Human Services et al. Guidance for Industry and FDA Staff: Class II Special Controls Guidance Document: Arrhythmia Detector and Alarm. (2003).
Apple Inc. Using Apple Watch for Arrhythmia Detection. (2018).
Parvinian, B., Scully, C., Wiyor, H., Kumar, A. & Weininger, S. Regulatory considerations for physiological closed-loop controlled medical devices used for automated critical care: food and drug administration workshop discussion topics. Anesth. Analg. 126, 1916–1925 (2018).
Allen, N. & Gupta, A. Current Diabetes Technology: Striving for the Artificial Pancreas. Diagnostics (Basel) 9, 31 (2019).
The 670G System - P160017. Available at: http://wayback.archive-it.org/7993/20170111141252/http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm522764.htm. (Accessed: 19th September 2019).
Watanabe, N. et al. Development and Validation of a Novel Cuff-Less Blood Pressure Monitoring Device. J Am Coll Cardiol Basic Trans. JACC: Basic to Translational Science 2, 631–642 (2017).
IEEE Standard for Wearable Cuffless Blood Pressure Measuring Devices. IEEE Std 1708-2014 1–38 (2014). https://doi.org/10.1109/IEEESTD.2014.6882122.
International Organization for Standardization & International Electrotechnical Commission. ANSI/AAMI/ISO 81060-2:2013: Non-invasive sphygmomanometers — Part 2: Clinical investigation of automated measurement type.
IEEE standard for software verification and validation. (Institute of Electrical and Electronics Engineers, 1998).
Kourtis, L. C., Regele, O. B., Wright, J. M. & Jones, G. B. Digital biomarkers for Alzheimer’s disease: the mobile/wearable devices opportunity. Npj Digit. Med. 2, 1–9 (2019).
Acknowledgements
We are grateful for input from Geoffrey S. Ginsburg MD, PhD on the language and processes used to evaluate the evidence base supporting the development and use of genetic tests to improve patient care and treatment. We are grateful for the support of many additional members of the Digital Medicine Society (DiMe) for providing expertize and insights on particular topics during the development of this work.
Author information
Authors and Affiliations
Contributions
Conceptualization: J.G., A.C., J.D. Analysis and writing: all authors made substantial contributions to the conception or design of the work, participated in drafting and revisions, and provided final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
This collaborative manuscript was developed as part of research initiatives led by the Digital Medicine Society (DiMe). All authors are members of DiMe. DiMe is a Massachusetts non-profit corporation with 501(c)(3) application pending. J.C.G. is a part-time employee of HealthMode, Inc. A.C. is founder of Elektra Labs. E.I. is an executive of Koneksa Health. J.P.B. is a full-time employee of Philips.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Goldsack, J.C., Coravos, A., Bakker, J.P. et al. Verification, analytical validation, and clinical validation (V3): the foundation of determining fit-for-purpose for Biometric Monitoring Technologies (BioMeTs). npj Digit. Med. 3, 55 (2020). https://doi.org/10.1038/s41746-020-0260-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41746-020-0260-4
This article is cited by
-
NiMBaLWear analytics pipeline for wearable sensors: a modular, open-source platform for evaluating multiple domains of health and behaviour
BMC Digital Health (2024)
-
Monitoring Activity and Gait in Children (MAGIC) using digital health technologies
Pediatric Research (2024)
-
Walk, talk, think, see and feel: harnessing the power of digital biomarkers in healthcare
npj Digital Medicine (2024)
-
Improved measurement of disease progression in people living with early Parkinson’s disease using digital health technologies
Communications Medicine (2024)
-
Decentralized clinical trials and rare diseases: a Drug Information Association Innovative Design Scientific Working Group (DIA-IDSWG) perspective
Orphanet Journal of Rare Diseases (2023)