Abstract
Developing characterization strategies to better understand nanoscale features in two-dimensional nanomaterials is of crucial importance, as the properties of these materials are many times driven by nanoscale and microscale chemical and structural modifications within the material. For the case of large area monolayer MoSe2 flakes, kelvin probe force microscopy coupled with tip-enhanced photoluminescence was utilized to evaluate such features including internal grain boundaries, edge effects, bilayer contributions, and effects of oxidation/aging, many of which are invisible to topographical mapping. A reduction in surface potential due to n-type behavior was observed at the edge of the flakes as well as near grain boundaries. Potential phase mapping, which corresponds to the local dielectric constant, depicted local biexciton and trion states in optically-active regions of interest such as grain boundaries. Finally, nanoscale surface potential and photoluminescence mapping was performed at several stages of oxidation, revealing that various oxidative states can be evaluated during the aging process. Importantly, all of the characterization performed in this study was non-destructive and rapid, crucial for quality evaluation of an exciting class of two-dimensional nanomaterials.
Similar content being viewed by others
Introduction
The unusual properties of two-dimensional (2D) materials are often generated not from a perfect crystalline lattice, but by the defects, grain boundaries, and other imperfections embedded within the structure. In 2D molybdenum diselenide (MoSe2), a van der Waals semiconductor with monolayer direct bandgap of 1.5 eV1, these inhomogeneities can range in size from as small as a selenium vacancy2,3,4 to microscale in the case of grain boundaries and locally oxidized domains5,6,7. While many researchers are pushing the limits of crystal perfection, the inhomogeneities and defects have proven beneficial in the cases of quantum emission from atomic vacancies8,9,10, electronic transport modification at grain boundaries11,12, and improved catalysis in sub-stoichiometric materials13. Often, these features are only observed via low-throughput techniques such as transmission electron microscopy or scanning tunneling microscopy14,15,16. To ensure implementation of 2D materials in desired applications including electronics, molecular sensors, and optoelectronics, new strategies to enable quantitative understanding of these defects using rapid, nondestructive characterization techniques will become increasingly necessary17,18. Many techniques also vary in instrument availability, cost, and difficulty, necessitating easier methods such as kelvin probe force microscopy (KPFM) and tip enhanced photoluminescence (TEPL), as is presented in this study.
Of the many subsets of nanoscale characterization methodologies available for 2D materials, scanning probe techniques routinely provide exceptional lateral resolution (~20 nm), nondestructive evaluation, and simplicity of use necessary to observe inhomogeneities in the 2D structure and chemistry. Atomic force microscopy, developed in the late 1980’s19, is the most common of these and enables rapid evaluation of nanoscale (~0.1 nm in height) topographical features to include layer thickness, grain boundaries, and even frictional properties in 2D materials20,21. KPFM22, a non-contact scanning probe technique, can allow for direct measurement of surface potential and work function at a similar scale. Using this technique, the surface potential of MoSe2 monolayers was shown to be highly thickness and environment-dependent, with an increase in work function with layer count due to interlayer screening effects23,24. Further surface potential and capacitance imaging of monolayer MoSe2 flakes reveal grain boundaries and edge effects, invisible to topographic mapping, exhibit a strong contribution to the localized surface potential25.
Optical analysis is fundamentally limited by the light diffraction limit (up to hundreds of nanometers for visible frequencies), which has prevented nanoscale mapping of optical signals and hence correlation of optical or excited state properties with topographic features. However, these limitations can be overcome by combining scanning probe techniques with spectroscopy. Tip-enhanced scanning probe techniques offers nanoscale-resolution imaging of optical signals, such as the Raman scattering and photoluminescence. The resolution offered is far-superior as compared to diffraction-limited far field imaging due to the deep sub-wavelength confinement of light by the plasmonic mode of the metal tip. The limiting resolution is ~20 nm, mainly defined by the tip diameter26. It has been used in the past to investigate the TEPL signal as a function of the number of layers as well as mapping of local strain, heterogeneity of emission and charged emission in transition metal dichalcogenides (TMDCs)27,28,29,30. The techniques’ ability to resolve the nanoscale optical emission properties has enabled experimental distinction of edge and basal plane “bulk” regions, aiding in the identification of 1D to 2D quantum confinement effect in TMDCs. For example, it was recently reported that an energetically disordering nanoscale region of ~300 nm width is present at the edges and grain boundaries in CVD grown MoS2, where the spectral median is blue shifted compared to basal plane “bulk” regions31. In another report, 1D MoS2 nanoribbons were synthesized and were found to emit at higher energies (blue shifted) as compared to 2D MoS2 when measured via TEPL32. In these bottom up synthesized MoS2 nanoribbons, the near field photoluminescence was measured in <200 nm width regions which fall entirely in the edge-boundary disordered PL quenched region as discussed above report. However, the systematic study on the spatial correlation between KPFM (surface potential) and TEPL is yet to be thoroughly investigated, and presents a new frontier in materials surface and near-surface characterization.
In this study, topographically-hidden features within large area MoSe2 flakes were evaluated using KPFM and correlated to local TEPL mapping, enabling a nondestructive quantitative evaluation of grain boundaries, edges, and oxidized regions within the structure. Both of these techniques were performed without the need to transfer the film from the growth substrate and are entirely non-destructive, unlike the case for tip-enhanced Raman spectroscopy, which requires transparent substrates33 or transfer to metallic films34,35. By evaluating the monolayer 2D materials on the native growth substrates, intrinsic nanoscale features of these exciting materials on silicon substrates can be evaluated independent of the influence of film transfer.
Results and discussion
Monolayer MoSe2 synthesis and characterization
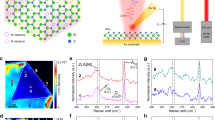
Large area monolayer MoSe2 flakes were synthesized directly onto a Si/SiO2 wafer through the vaporization of MoO3 and selenium powder at elevated temperatures, as schematically depicted in Fig. 1a and described in previous works36,37. The resultant films were primarily monolayer with some bilayer regions and exhibited lateral flake sizes in excess of 25 µm with characteristic A1g (241.1 cm−1) and E12g (287.1 cm−1) peaks in the Raman spectrum (Fig. 1b). Initial Raman mapping of a microscale flake in Fig. 1c revealed apparent uniform structure at the resolution of the far-field imaging technique (~500 nm). Powder vaporization synthesis of 2D materials has been known to result in many features and inhomogeneities on and within the surface including bilayers and grain boundaries. These features are invisible in the initial Raman mapping but are clearly present in the topographical and KPFM maps as shown in Fig. 1d, e, respectively.
Scanning probe topography and KPFM of the monolayer flakes are not limited by the same resolution as Raman spectroscopy, but at times the data can be difficult to translate into meaningful quantitative information. The topographic mapping of the MoSe2 flakes in Fig. 1d easily identifies bilayer triangles within the flake but is blind to chemical changes until they are very advanced to the point of structural modification of the crystal or creation of oxide complexes38. Kelvin probe force microscopy mapping in Fig. 1e clearly depicts regions of varying chemical potential on the surface, with obvious deviations from the bulk flake occurring at both the edges and the bilayer regions, revealing that these techniques can successfully identify features beyond the visible diffraction limit.
KPFM measures the contact potential difference (CPD)39 to produce a relative work function map of the surface. It is well-known that KPFM can see changes in work function in 2D materials due to a change in layer number, grain boundaries, and chemical changes like defect densities23,40. The phase map is less well-studied, but can generally be considered analogous to surface dielectric properties (see Supplementary Information: KPFM Operation and Theory)26,39,40. Unlike CPD, the phase signal is blind to surface charging, allowing resolution of finer features where charge accumulates.
Investigating nanoscale heterogeneities with KPFM
To correlate the topographical features to various chemical and dielectric states in the material, topographical mapping (Fig. 2a), KPFM (Fig. 2b, c), and phase mapping (Fig. 2d) were performed sequentially on the same flake area. Clearly, the chemical potential and dielectric properties of the edge, monolayer and bilayer regions result in a clear contrast from the rest of the flake. To further evaluate this, line scans taken from Fig. 2b internal grain boundary, and bilayer sites as well as the corresponding height profiles are shown in Fig. 2c, respectively. The edge effect is visible in Fig. 2b, but an enhanced image and line scan of an fully unoxidized flake is shown in Fig. 2e, f, respectively. The edge of the flake shows a small rise (~50 mV) in work function within a 300 nm distance from the boundary of the flake before dropping off to the substrate value of −400 mV below the flake. We attribute this small rise in edge potential before drop-off to the molybdenum-rich MoSe2 chemistry observed to occur due to the growth process, which results in local n-doping at the edges41,42. The width of this region indicates the formation of a depletion region around the potential differential as verified in conducting AFM studies in 2D materials43. The phase map excludes this region, but has relatively low contrast (Supplementary Fig. 1) compared to the potential map. The relatively slow drop in potential beyond the edge of the flake (on the order of 500 nm) is dominated by charge accumulation in the SiO2 substrate. A significant drop in potential (~50 mV) was observed at the grain boundary, which is invisible in the topographic map but very apparent in surface phase map (Fig. 2b). This is a very favorable feature of utilizing KPFM to evaluate 2D materials in that chemical changes and features in the film are easily observable, in this case even when structurally they would otherwise be indistinguishable. Additionally, bilayers interrogated with KPFM showed a rise in surface potential of nearly 100 mV. It was determined, and will be discussed later, that the majority of this top-most plane on bilayer flakes was oxidized at the point of taking this measurement, which affects the relative grain boundary and bilayer potentials as the flakes age and resulting in an increase in surface potential when oxidized.
a Topography AFM image, b KPFM potential map with scale bars equal to 5 μm, c line scans of edge grain boundary (blue line in b) and bilayer (yellow line in b) with associated height scans, d KPFM phase map with scale bars equal to 5 μm, e height and KPFM potential of enhanced edge of new flake with scale bar equivalent to 1 μm, and f line scans of height and potential in e.
Due to their atomically-thin structure, the electronic and optical properties of single layer TMDCs can be significantly influenced by elements of the surrounding environment such as chemical dopants, fixed charges and nature of the dielectric medium44,45,46. As discussed above, the KPFM phase characterization reflects the dielectric property of materials and it therefore impacts optical properties of the layers. Hence, to further correlate KPFM to optical properties, specifically luminescent properties, we perform TEPL measurements on the MoSe2 flakes.
Correlation of nanoscale features using tip-enhanced photoluminescence
We first focus on a region composed of monolayer MoSe2 and also bilayer parts as shown in the topographical map in Fig. 3a. With increased aging of the flake, a natural hump emerges near the internal grain-boundary where it was invisible initially in topography. This can be attributed to physisorption or chemisorption of foreign molecules due to the chemically active nature of the surface at this grain boundary47,48. Corresponding KPFM phase mapping of the grain boundary shows a lower phase value than basal plane, similar to the result at Fig. 2b. Corresponding spatial variation of TEPL peak position is shown in Fig. 3c. Overall, the PL peak positions throughout the flakes are redshifted compared to the peak positions of previously reported PL emission of MoSe2 at ~800 nm25, which is attributed to oxygen adsorption on the flakes. It has been widely reported that oxygen easily adsorbs to chalcogen vacancy sites in TMDCs and acts as a p-dopant49,50. This results in an increase in the number of positively charged excitons (trions) rather than neutral excitons and hence the PL is red-shifted. Taking this into account, we speculate that the as-prepared sample has stoichiometric composition variation throughout the flakes. This presumed composition variation is likely composed of differences in selenium vacancy concentration, and hence the variance in the extent of subsequent oxygen chemisorption on the surface. Due to the varying degrees of oxidation, the surface in Fig. 3c shows four different regions of varying emission spectra denoted as Region 1–4, corresponding to a grain boundary, highly oxidized basal plane, basal plane and edge. Each region emits a peak PL at 818.2 nm (Region 1), 820.0 nm (Region 2), 817.3 nm (Region 3), and 814.0 nm (Region 4) on average.
a Topography b KPFM phase, and c near-field PL wavelength mapping of monolayer MoSe2, d Energy diagram of exciton, trion, and biexciton state, e representative deconvoluted averaged PL Spectrum of Region 1, f Near field PL mapping of AX−/+ (~1.520 eV) and g AXX (~1.490 eV), and h Areal PL contribution of trion and biexciton at Region 1–4. All scale bars are equivalent to 3 µm.
KPFM phase and TEPL maps were compared to elucidate more quantitative information regarding the microstructure and chemistry in this study. Although each region is not clearly distinguished in topography (Fig. 3a), KPFM phase changes with time and the corresponding TEPL peak position map (Fig. 3c) resembles the phase map. The KPFM phase can be directly correlated to the static dielectric constant of the sample medium. Therefore, upon comparing Fig. 3b, c, it is evident that the static dielectric constant varies even in a topographically homogeneous single flake from the chemisorption of oxygen and has a critical role in the resultant luminescent properties. More specifically, at Region 2, a dimmer KPFM phase region appears at the center and the peak position for the corresponding wavelength is far red-shifted compared to the other region. This result can be attributed to oxygen chemisorption on to MoSe2 flake and alteration of the dielectric environment on the flake. In agreement with a previous study reporting shifts in the PL peak position with different surrounding dielectric media due to reduced dielectric screening45, the corresponding binding energy becomes stronger and its PL peak position is red-shifted. The dimmer KPFM phase region is therefore characterized by a comparatively smaller dielectric constant and that local variation of KPFM phase suggests partial oxidation or chemisorption. More details of oxidation evolution are discussed further below.
Figure 3c further suggests that our observed PL emission is even more redshifted than that of trion (813 nm) and neutral exciton (800 nm, 1.55 eV) PL normally observed25, leading to the consideration of the potential occurrence of higher order excited states in addition to the trion. Prior reports suggest that biexciton formation in bilayer MoSe2 occurs even at room temperature and further confirmed that biexcitons are formed by the combination of a trion and an excess free carrier (i.e. an electron or a hole)51. A key requirement for stable biexciton formation is high exciton density as well as a binding energy greater than the room temperature thermal energy. One main difference in previously published results describe biexcitonic emission in both monolayer as well as bilayer MoSe2. We attribute this result to tip-mode enhancement of PL which causes plasmonic enhancement at vicinity of the tip apex due to the Purcell effect52. Since interaction with the tip creates excess carriers in an extremely localized region due to the small tip radius (~20 nm), exciton density in the region is dramatically higher as opposed to far-field excitation at same total power, hence the formation of stable biexcitons at room temperature. The stable biexciton formation in our case is supported by the prior report about resonant excitation of CsPbBr3 perovskite nanoplate inside the Ag nanowire-Ag film plasmonic nanogaps53, which reported that the necessary exciton occupancy for excitation was significantly reduced by ~106 times from the plasmonic gap. We rule out the possibility of detrimental influence of the Purcell effect on MoSe2 since plasmonic resonators are well known for large and reproducible enhancement of spontaneous emission52,54,55,56. For example, recent reports on time varying series of tip-enhanced photoluminescence of WSe2 by repeated engaging and disengaging the tip has proven Purcell enhancement of PL unambiguously. The same work also showed that WSe2 monolayer is stable under frequent exposure to high-electric field induced by Purcell effect52. Further, biexciton emission due to such high intensity laser has thus far only been observed in the far-field51,57. The biexciton emission in our case from a near field signal is indicative of a cumulative effect of Purcell enhancement and oxygen induced doping. Considering this, we evaluated the trion/biexciton contribution of the TEPL spectrum. Figure 3d demonstrates energy states of the ground exciton (A1s), trion (AX−/+) and biexciton (AXX) energy band. Since the biexciton originates from a trion, its energy state is lower than that of the trion and separated by its binding energy51. Based on the energy level, the PL spectrum can be deconvoluted into AX−/+ (red) and AXX (green) peaks, as shown in Fig. 3e and supporting information (Supplementary Fig. 2). Note that the neutral exciton signal (800 nm) is negligible in comparison to AX−/+ and AXX contribution as we assumed before. Figure 3f, g displays the PL intensity from AX−/+ and AXX, respectively, where AX−/+ emission is uniform over the entire flake while the PL intensity from AXX is particularly high in Region 2. This suggests that the AXX are readily formed at the oxidized region because of low dielectric screening (dimmer KPFM phase), which contributes to enhancement of the binding energy. This observed trend is in agreement with previous reports about large binding energy of biexciton at nanomaterials such as two dimensional PbI4 under small dielectric medium58.
Likewise, near the flake edge (Region 4) and the grain boundary (Region 1), the exciton has higher binding energy because the dielectric constant is relatively lower than that of the symmetric, defect free basal plane (Region 2, 3 in Fig. 3b). Interestingly, we found the PL intensity at the edge and grain boundary are much stronger than that at the basal plane. The large variations in PL of edges vs basal plane regions have been made previously in both CVD grown and exfoliated flakes, and many explanations have been pointed out for this including presence of oxidation of the edge site31,32,47 to local disorder to potential confinement of excitations near the edge. However, these results are not sufficient to explain this solely by oxidation or due to the structural and electronic property difference between basal plane and the edge. As indicated in Fig. 3b, the KPFM phase gradient is present from the basal plane to the edge or the grain boundary. We believe the dielectric gradient can be a driving force to funnel the exciton in the vicinity of these line defects because the low dielectric medium offers stronger oscillation bond in the electron-hole pair and more stable exciton formation59. It is supported by the fact that the line cut of PL map indicates depletion of PL emission close to both edge and basal plane (Supplementary Fig. 3). Interestingly, however, A1s emission was negligible at the edge and grain boundary despite the low dielectric screening. This is because the biexciton state is readily formed under high electric field. According to Javey et al.60, recombination rate of the trion and biexciton (1015 cm−2 s−1) on MoS2 exceeds that of exciton under high generation rate (1019 cm−2 s−1), which is achieved by high intensity of the laser excitation. In addition, electrostatic p-doping facilitates a high recombination rate (1015 cm−2 s−1) at even lower generation rates (1016 cm−2 s−1). Moreover, the dielectric gradient driven exciton funneling effect can further enhance the trion and biexcitonic emission rather than neutral exciton at the edge and the grain boundary due to the lower dielectric constant at the edge and grain boundary as seen in Fig. 3b. Note that biexcitons in our case are formed by reaction between trions and excess free carriers rather than two neutral excitons51. Since there are several e–h pairs generated in the vicinity of tip mode due to the diffraction limited spot of the laser, they can act as a reservoir of free charges that react/combine with trions and create high density biexciton population within the cavity. Another plausible explanation is that line defects and edges exhibit an out-of-plane dipole for the exciton. This would result in stronger coupling with the tip and stronger Purcell effect, leading to higher PL intensity during TEPL measurement59. In addition, trion and biexcitonic emission at non-oxidized grain boundary are suppressed at Fig. 3f, g. This can be explained by a varition in the density of the trion. In this case, the existance of a non-oxidized grain boundary creates a low density of trions due to the fact that it is not p-doped. As we mentioned above, biexcitons are created by combining trion and charge carrier rather than two neutral bound or free excitons. Hence the trion and biexcitonic emission is relatively small although the grain boundary has low dielectric medium. This result further verifies oxidation is important step to create biexcitonic emission by creating trion states.
The graph in Fig. 3h reveals the PL intensity of both the AX−/+ and AXX at Region 1–4. The main contribution of the PL arises from AX−/+. Since AX−/+ is a charged exciton, stronger AX−/+ emission reflects higher concentration, with oxygen chemisorption affecting the basal plane more strongly than the edge. Correspondingly, AXX emission tracks the spatial variation of the AX−/+ emission at the edge while it does not on the basal plane. Note that the intensity of AXX is smaller than that of AX−/+ because the AXX is formed by combining AX−/+ and an excess carrier. One distinguishing feature of the edge and grain boundary is that the biexciton intensity is unusually high at the grain boundary (Region 1) while low at the edge. This can be further explained by the funneling effect as discussed above.
Tracking oxidation and aging
Oxidation was first observed on flakes that were a few months old and subjected to prolonged exposure to a combination of glovebox storage and ambient air. The spot region of oxidation on the top and left image of the flake in Fig. 3a reveals an approximately 30 mV reduction in surface potential relative to the adjacent unoxidized basal plane. Over the course of the 6 months, the regions on two different flakes were observed to grow in both KPFM and TEPL, until they filled nearly the entire flakes (Fig. 4a–c). Since oxidation p-dopes the MoSe2 flake, KPFM potential is lowered as the workfunction of MoSe2 increases. It is further verified from the PL peak position mapping, showing that the lower potential region emits PL at lower energy peak position. Interestingly, the oxidation does not start at the grain boundary but at the basal plane. Previous reports state that the grain boundary and edge of the flake is vulnerable to oxidation, because the unterminated molybdenum edges are more chemically active than the basal plane47. This result is in contrast to the previous reports and this phenomenon was repeatedly observed on multiple flakes (Fig. 3c and 4a). As the flake oxidized, the potential value changes throughout the flake, resulting in a decrease in potential by 48 mV as indicated in Fig. 4b. Additionally, it was clear that the oxidation influenced the bilayer region differently than the monolayer regions. Interestingly, bilayers atop the oxidized region showed a potential higher even than the basal plane, whereas bilayers on the basal plane showed a much lower potential consistent with literature values in pristine TMD flakes23,61,62. This is hypothesized to be due to potentially different oxidative states within the material such as chemisorption of oxygen to the bilayer rather than physisorption. It was reported that chemisorption of oxygen influences MoS2 to be n-doped while physisorption induces p-doping, which was supported by first-principle calculation stating that oxygen chemisorption results in conduction band minimum shift downward with respect to fermi level63. On the other hand, physisorption is favorable at monolayer region, leading to p-doping and consequently lower potential. The bilayer potential is significantly changed after oxidation (ΔV = +100 mV) while that of monolayer (ΔV = −40 mV) is relatively small. We attribute this to chalcogen p-orbital perturbation due to the adsorption of oxygen. The band structure of MoSe2 is composed of Γ(Q) states in the band dominated by p-orbital of Se and K valley states dominated by d-orbital of Mo. The monolayer band structure is mostly determined by K states composed of the d-orbitals of Mo. In the case of bilayer MoSe2, the Γ(Q) states are shifted due to the interlayer coupling, while the K states are almost constant64. Since the adsorbates directly contact to the chalcogen layer and couples with p-orbitals, bilayer MoSe2 exhibits a large change in CPD after oxidation compared to the monolayer. Note that the time-dependent CPD change is unlikely to be related to substitution of Se site by oxygen since forming SeO2 is thermodynamically unfavorable in the basal plane65. Hence the CPD changes result from vacancies formed during material synthesis, and the bilayer is eventually further distinguished from monolayer in the case of oxidized flakes as indicated in Fig. 4b. This localized oxidative and chemical state within the MoSe2 flakes reinforces the necessity to further develop nanoscale characterization techniques for these unique and exciting class of nanomaterials.
Characterization of large area monolayer MoSe2 flakes by surface potential mapping, phase mapping, and tip-enhanced photoluminescence evaluation reveal many nanoscale features invisible to topographical mapping, and provide insight into local chemical and excitonic states within the material. Observed increase in surface potential approaching 300 nm from the edge of the flake reveal n-type doping due to substoichiometric film growth. Internal grain boundaries with no visible height increase are clearly observed in KPFM from chemical modifications and drive a local surface potential decrease of nearly 50 mV. Correlated KPFM phase mapping and TEPL track the local dielectric properties to both biexciton and trion states, critical to optimizing and understanding future optical applications. Finally, by performing both surface potential evaluation and optical nanoprobes, tracking of oxidation over time can allow for understanding the influence of environmental conditions on these important features. As these nanoscale to microscale defects, grain boundaries, and surface states will drive much of the observed properties of 2D materials, the non-destructive characterization provided herein provides for another tool for scientists and engineers looking towards applications of this exciting class of nanomaterials.
Methods
Monolayer MoSe2 synthesis
Chemical vapor deposition of MoSe2 films were performed in a Thermo Scientific Lindberg Blue Mini Mite tube furnace. All reagents were obtained from Millipore Sigma. MoO3 precursor is placed in a ceramic boat with a cleaned Si/SiO2 substrate suspended face down above MoO3 located at the center of furnace, where maximum temperature reaches 750 °C. A second ceramic boat containing Se powder is located upstream where maximum temperature reaches 500 °C. After 1 min of purging, the furnace is heated to 750 °C from room temperature in 20 min and kept at the reaction temperature for 5 min. The furnace is cooled unassisted to room temperature resulting in triangle films of MoSe2. Throughout the entire process, N2 is continuously streamed into the furnace at a rate of 150 sccm. During the reaction time, a mixture of H2 and Ar (10% H2) is streamed into the furnace at a rate of 20 sccm and shut off once cooling begins.
KPFM characterization
KPFM measurements were performed with a Bruker Dimension Icon using PFQNE-AL probe tips. The tips were calibrated against an Al/Au standard. Data was normalized to the basal plane of the flake. To quantify the potential, the potential of select areas corresponding to features of interest was exported to OriginPro and fit to a Gaussian curve. The peak of this fit was then selected as a data point for that feature. KPFM measurement conditions were adjusted for each sample based on roughness and adhesion considerations. The lift height was between 25 and 35 nm. To compensate for charging in the flake, KPFM potential maps were normalized to the basal plane and line scans of relevant features were performed. For more information on KPFM characterization, see Supplementary Methods.
Tip-enhanced photoluminescence (TEPL) characterization
TEPL characterizations were performed in LabRam-Nano AFM-Raman systems (Horiba Scientific). A 633 nm laser was focused on Au-coated AFM tip (Omni TERS Probe) and the signal was collected by 100 g/mm grating. The spectra were averaged over the selected region covering at least 100 × 100 pixels with each pixel size of 80 × 80 nm2.
Data availability
All data generated or analyzed during this study are available upon request to the corresponding authors. The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Zhang, Y. et al. Direct observation of the transition from indirect to direct bandgap in atomically thin epitaxial MoSe2. Nat. Nanotechnol. 9, 111–115 (2014).
Meng, Y. et al. Repairing atomic vacancies in single-layer MoSe2 field-effect transistor and its defect dynamics. npj Quantum Mater. 2, 16 (2017).
Mahjouri-Samani, M. et al. Tailoring vacancies far beyond intrinsic levels changes the carrier type and optical response in monolayer MoSe2-x crystals. Nano Lett. 16, 5213–5220 (2016).
Lehtinen, O. et al. Atomic scale microstructure and properties of se-deficient two-dimensional MoSe2. ACS Nano. 9, 3274–3283 (2015).
Lin, Z. et al. Defect engineering of two-dimensional transition metal dichalcogenides. 2D Mater. 3, 022002 (2016).
Hu, Z. et al. Two-dimensional transition metal dichalcogenides: Interface and defect engineering. Chem. Soc. Rev. 47, 3100–3128 (2018).
Komsa, H. P. et al. Two-dimensional transition metal dichalcogenides under electron irradiation: defect production and doping. Phys. Rev. Lett. 109, 035503 (2012).
Zhang, S. et al. Defect structure of localized excitons in a WSe2 monolayer. Phys. Rev. Lett. 119, 046101 (2017).
Schwarz, S. et al. Electrically pumped single-defect light emitters in WSe2. 2D Mater. 3, 025038 (2016).
Dang, J. et al. Identifying defect-related quantum emitters in monolayer WSe2. npj 2D Mater. Appl. 4, 2 (2020).
Majee, A. K., Foss, C. J. & Aksamija, Z. Impact of mismatch angle on electronic transport across grain boundaries and interfaces in 2D materials. Sci. Rep. 7, 16597 (2017).
Mesaros, A., Papanikolaou, S., Flipse, C. F. J., Sadri, D. & Zaanen, J. Electronic states of graphene grain boundaries. Phys. Rev. B 82, 205119 (2010).
Truong, Q. D. et al. Defect-rich exfoliated MoSe2 nanosheets by supercritical fluid process as an attractive catalyst for hydrogen evolution in water. Appl. Surf. Sci. 505, 144537 (2020).
Huang, X., Zeng, Z. & Zhang, H. Metal dichalcogenide nanosheets: preparation, properties and applications. Chem. Soc. Rev. 42, 1934–1946 (2013).
Xie, L. M. Two-dimensional transition metal dichalcogenide alloys: preparation, characterization and applications. Nanoscale 7, 18392–18401 (2015).
Manzeli, S., Ovchinnikov, D., Pasquier, D., Yazyev, O. V. & Kis, A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2, 17033 (2017).
Liu, X. & Hersam, M. C. 2D materials for quantum information science. Nat. Rev. Mater. 4, 669–684 (2019).
Glavin, N. R. et al. Emerging applications of elemental 2D materials. Adv. Mater. 32, 1904302 (2020).
Binnig, G., Quate, C. F. & Gerber, C. Atomic force microscope. Phys. Rev. Lett. 56, 930 (1986).
Lee, C. et al. Frictional characteristics of atomically thin sheets. Science 328, 76–80 (2010).
Carpick, R. W. & Salmeron, M. Scratching the surface: fundamental investigations of tribology with atomic force microscopy. Chem. Rev. 97, 1163–1194 (1997).
Nonnenmacher, M., O’Boyle, M. P. & Wickramasinghe, H. K. Kelvin probe force microscopy. Appl. Phys. Lett. 58, 2921 (1991).
Borodin, B. R. et al. Kelvin probe microscopy of MoSe2 monolayers on graphene. J. Phys. Conf. Ser. 1124, 081031 (2018).
Feng, Y. et al. In situ visualization and detection of surface potential variation of mono and multilayer MoSe2 under different humidities using Kelvin probe force microscopy. Nanotechnology. 28, 295705 (2017).
Smithe, K. K. H. et al. Nanoscale heterogeneities in monolayer MoSe2 revealed by correlated scanning probe microscopy and tip-enhanced Raman spectroscopy. ACS Appl. Nano Mater 1, 572–579 (2018).
Atkin, J. et al. Nano-optical imaging and spectroscopy of order, phases, and domains in complex solids. Adv. Phys. 61, 745–842 (2012).
Su, W., Kumar, N., Mignuzzi, S., Crain, J. & Roy, D. Nanoscale mapping of excitonic processes in single-layer MoS2 using tip-enhanced photoluminescence microscopy. Nanoscale. 8, 10564–10569 (2016).
Kim, Y., Lee, Y., Kim, H., Roy, S. & Kim, J. Near-field exciton imaging of chemically treated MoS2 monolayers. Nanoscale. 10, 8851–8858 (2018).
Park, K. D. et al. Hybrid tip-enhanced nanospectroscopy and nanoimaging of monolayer WSe2 with local strain control. Nano Lett. 16, 2621–2627 (2016).
Sahoo, P. K. et al. Probing nano-heterogeneity and aging effects in lateral 2D heterostructures using tip-enhanced photoluminescence. Opt. Mater. Express. 9, 1620–1631 (2019).
Bao, W. et al. Visualizing nanoscale excitonic relaxation properties of disordered edges and grain boundaries in monolayer molybdenum disulfide. Nat. Commun. 6, 1–7 (2015).
Chowdhury, T. et al. Substrate-directed synthesis of MoS2 nanocrystals with tunable dimensionality and optical properties. Nat. Nanotechnol. 15, 29–34 (2020).
Kato, R., Umakoshi, T., Sam, R. T. & Verma, P. Probing nanoscale defects and wrinkles in MoS2 by tip-enhanced Raman spectroscopic imaging. Appl. Phys. Lett. 114, 073105 (2019).
Krayev, A. et al. Dry transfer of van der Waals crystals to noble metal surfaces to enable characterization of buried interfaces. ACS Appl. Mater. Interfaces. 11, 38218–38225 (2019).
Jariwala, D. et al. Nanoscale doping heterogeneity in few-layer WSe2 exfoliated onto noble metals revealed by correlated SPM and TERS imaging. 2D Mater. 5, 035003 (2018).
Najmaei, S. et al. Vapour phase growth and grain boundary structure of molybdenum disulphide atomic layers. Nat. Mater. 12, 754–759 (2013).
Wang, X. et al. Chemical vapor deposition growth of crystalline monolayer MoSe2. ACS Nano 8, 5125–5131 (2014).
Zhu, H. et al. Remote plasma oxidation and atomic layer etching of MoS2. ACS Appl. Mater. Interfaces 8, 19119–19126 (2016).
Melitz, W., Shen, J., Kummel, A. C. & Lee, S. Kelvin probe force microscopy and its application. Surf. Sci. Rep. 66, 1–27 (2011).
Qiao, Q. et al. Evolution of metastable defects and Its effect on the electronic properties of MoS2 films. Sci. Rep. 8, 1–10 (2018).
Kong, D. et al. Synthesis of MoS2 and MoSe2 films with vertically aligned layers. Nano Lett. 13, 1341–1347 (2013).
Tsai, C., Chan, K., Abild-Pedersen, F. & Norskov, J. K. Active edge sites in MoSe2 and WSe2 catalysts for the hydrogen evolution reaction: a density functional study. Phys. Chem. Chem. Phys. 16, 13156–13164 (2014).
Giannazzo, F. et al. Direct probing of grain boundary resistance in chemical vapor deposition-grown monolayer MoS2 by conductive atomic force microscopy. Phys. Status Solidi 14, 1–6 (2020).
Mouri, S., Miyauchi, Y. & Matsuda, K. Tunable photoluminescence of monolayer MoS2 via chemical doping. Nano Lett. 13, 5944–5948 (2013).
Lin, Y. et al. Dielectric screening of excitons and trions in single-layer MoS2. Nano Lett. 14, 5569–5576 (2014).
Prins, F., Goodman, A. J. & Tisdale, W. A. Reduced dielectric screening and enhanced energy transfer in single- and few-layer MoS2. Nano Lett. 14, 6087–6091 (2014).
Rong, Y. et al. Controlled preferential oxidation of grain boundaries in monolayer tungsten disulfide for direct optical imaging. ACS Nano 9, 3695–3703 (2015).
Longo, R. C. et al. Intrinsic air stability mechanisms of two-dimensional transition metal dichalcogenide surfaces: basal versus edge oxidation. 2D Mater. 4, 025050 (2017).
Tongay, S. et al. Broad-range modulation of light emission in two-dimensional semiconductors by molecular physisorption gating. Nano Lett. 13, 2831–2836 (2013).
Shepard, G. D. et al. Trion-species-resolved quantum beats in MoSe2. ACS Nano 11, 11550–11558 (2017).
Pei, J. et al. Excited state biexcitons in atomically thin MoSe2. ACS Nano 11, 7468–7475 (2017).
Park, K. D., Jiang, T., Clark, G., Xu, X. & Raschke, M. B. Radiative control of dark excitons at room temperature by nano-optical antenna-tip Purcell effect. Nat. Nanotechnol. 13, 59–64 (2018).
Scherübl, Z. et al. Observation of spin–orbit coupling induced Weyl points in a two-electron double quantum dot. Commun. Phys. 2, 108 (2019).
Zhang, Y. et al. Simultaneous surface-enhanced resonant Raman and fluorescence spectroscopy of monolayer MoSe2: determination of ultrafast decay rates in nanometer dimension. Nano Lett. 19, 6284–6291 (2019).
Huang, J., Akselrod, G. M., Ming, T., Kong, J. & Mikkelsen, M. H. Tailored emission spectrum of 2D semiconductors using plasmonic nanocavities. ACS Photonics 5, 552–558 (2018).
Mukherjee, B. et al. Exciton emission intensity modulation of monolayer MoS2 via Au plasmon coupling. Sci. Rep. 7, 1–11 (2017).
Yong, C. K. et al. Biexcitonic optical Stark effects in monolayer molybdenum diselenide. Nat. Phys. 14, 1092–1096 (2018).
Ishihara, T., Hong, X., Ding, J. & Nurmikko, A. V. Dielectric confinement effect for exciton and biexciton states in PbI4-based two-dimensional semiconductor structures. Surf. Sci. 267, 323–326 (1992).
Raja, A. et al. Dielectric disorder in two-dimensional materials. Nat. Nanotechnol. 14, 832–837 (2019).
Lien, D. H. et al. Electrical suppression of all nonradiative recombination pathways in monolayer semiconductors. Science 364, 468–471 (2019).
Choi, S. H., Shaolin, Z. & Yang, W. Layer-number-dependent work function of MoS2 nanoflakes. J. Korean Phys. Soc. 64, 1550–1555 (2014).
Wang, X. et al. Defect heterogeneity in monolayer WS2 unveiled by work function variance. Chem. Mater. 31, 7970–7978 (2019).
Qi, L., Wang, Y., Shen, L. & Wu, Y. Chemisorption-induced n-doping of MoS2 by oxygen. Appl. Phys. Lett. 108, 063103 (2016).
Liu, H. et al. Line and point defects in MoSe2 bilayer studied by scanning tunneling microscopy and spectroscopy. ACS Nano 9, 6619–6625 (2015).
Pető, J. et al. Spontaneous doping of the basal plane of MoS2 single layers through oxygen substitution under ambient conditions. Nat. Chem. 10, 1246–1251 (2018).
Acknowledgements
N.G. acknowledges support of Air Force Office of Scientific Research grant FA9550-19RYCOR050. D.J. and K.J. acknowledge primary support for this work by the Air Force Office of Scientific Research (AFOSR) FA9550-21-1-0035 and FA2386-20-1-4074 and partial support from U.S. Army Research Office under contract number W911NF-19-1-0109. D.J. also acknowledges partial support from National Science Foundation (DMR-1905853) and from Penn Engineering Start-up funds. The near-field work was carried out at the Singh Center for Nanotechnology at the University of Pennsylvania which is supported by the National Science Foundation (NSF) National Nanotechnology Coordinated Infrastructure Program grant NNCI-1542153. J.L. and C.N. acknowledges support from the Welch foundation grant C-1716 and NSF I/UCRC Center for Atomically Thin Multifunctional Coatings (ATOMIC) under award #IIP-1539999.
Author information
Authors and Affiliations
Contributions
All authors contributed to this study. D.M. and K.J. contributed equally to the manuscript, with D.M. performing the KPFM analysis and K.J. the PL mapping. C.N. and J.L. synthesized and characterized the MoSe2 flakes. C.M. contributed intellectually and D.J. and N.G. were the thought leaders in the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moore, D., Jo, K., Nguyen, C. et al. Uncovering topographically hidden features in 2D MoSe2 with correlated potential and optical nanoprobes. npj 2D Mater Appl 4, 44 (2020). https://doi.org/10.1038/s41699-020-00178-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41699-020-00178-w
This article is cited by
-
Bowing-alleviated continuous bandgap engineering of wafer-scale WS2xSe2(1-x) monolayer alloys and their assembly into hetero-multilayers
NPG Asia Materials (2022)
-
Light–matter coupling in large-area van der Waals superlattices
Nature Nanotechnology (2022)