Abstract
The development of synthetic two-dimensional crystalline polymers (2DCPs), such as 2D covalent-organic polymers and 2D metal-organic polymers, is receiving increasing attention due to their intriguing chemistry and unique properties, as well as potential role in wide ranging applications, such as electronics, sensing, catalysis, separation, and energy storage and conversion. Complementary to the top-down exfoliation towards the preparation of 2DCPs, bottom-up interface-assisted synthesis is advantageous in the 2D dynamic arrangement of the molecules or precursors, offering the chance to generate ultra-thin structures with large lateral sizes. This article provides guidelines on the preparation of free-standing, single-layer, or multi-layer 2DCPs via liquid-interface-assisted synthesis, mainly involving polymerization at the air–water and liquid–liquid interfaces, as well as the Langmuir-Blodgett method. Insight into the advantages and challenges of synthesis strategies and chemistry methodologies are provided for the future development of interfacial synthesis of 2DCPs with diverse structural and functional control.
Similar content being viewed by others
Introduction
Synthetic two-dimensional crystalline polymers (2DCPs) are categorized as one to a few atom-unit or monomer-unit thin networks with well-defined periodicity along two orthogonal directions via strong covalent or coordination bonds, generally including 2D polymers (2DPs),1,2,3 2D covalent-organic frameworks (2D COFs)4,5,6 and 2D metal-organic frameworks (2D MOFs).7,8 In these cases, the criteria of 2DPs is increasingly accepted as single-atom/monomer-thick, covalent networks with planar and highly ordered structures. COFs are a class of organic crystalline porous polymers that allow the atomically precise integration of organic units into extended structures with periodic skeletons and ordered nanopores by strong covalent bonds. Generally, 2D COFs are van der Waals (vdW) layer-stacked structures while a single-layer 2D COF can be regarded as a 2DP. 2D MOFs are crystalline, porous coordination polymers made by connecting inorganic metal ions or clusters with ditopic or polytopic organic ligands and featured with layered structures with strong in-plane coordination but weak out-plane interactions. As a major class of organic 2D materials, their structures (pore), properties, and functions can be tuned to a much higher degree, due to the abundant monomer and linkage chemistries that can be employed. Thus, tailor-made 2DCPs have been achieved with π-conjugated structures, electron-donating/-accepting groups, catalytic, sensing, magnetic, electroactive, and photoactive properties, and adsorption and separation functions, etc. Exceptionally, graphene is also regarded as a naturally available 2D crystalline polymer.9 In comparison with graphene, the electronic band gaps of these synthetic 2DCPs can be finely adjusted by varying monomers and linkages at the molecular level. Despite of the various advantages, the controlled synthesis of 2DCPs with tunable size and thickness in a large scale remains challenging.
By far, several strategies have been attempted. On the one hand, pioneering works on 2D poly(m-phenylene), covalent assemblies of porphyrin and thiophene, dynamic covalent polyimine networks, and metal-organic networks have been reported via the on-surface synthesis.10,11,12,13,14,15 However, the mobility of monomers on the surface is limited and only small domain sizes have been obtained. Moreover, a transfer of such metal surface-binding polymer networks is a complicated issue. On the other hand, despite of the recent progress in the synthesis of ultra-thin nanosheets via the top-down exfoliation of layered COFs, MOFs, and polymer crystals,16,17,18,19 the broad distribution of sheet thickness and lateral size, as well as low structure integrity and high tendency of re-stacking are unexpected.
To overcome these disadvantages associated with the on-surface synthesis and solution exfoliation protocols, air–water and liquid–liquid interfaces assisted synthesis have been recently introduced,20,21,22,23,24,25,26 which enables the successful preparation of 2DCPs. Such liquid-interface-assisted synthesis strategies offer the possibility to overcome the limited diffusion of monomers, and the interface acts as the template for the 2D-confined polymerization. The obtained 2DCPs present expected advantages, such as large-area up to cm2, free-standing feature, single-layer or multi-layer thickness, porous skeletons, high structural perfection, and high mechanical stability.20,22,23,25,26 Moreover, such 2DCPs can be directly transferred from the interfaces to various solid surfaces for further characterization and device fabrication.22,23,24,25,26 In this Perspective article, we include representative examples and highlight the roles of air–water and liquid–liquid interfaces in the successful control of 2D structure and morphology. We will not cover the comprehensive literature; there are relevant reviews for various synthetic 2DCPs.2,3,5,6,8,27 Our purpose is to provide a succinct summary of the synthesis strategies and chemistry methodologies of previous work, some pressing and important challenges, as well as a few characterization methods, which could be helpful for those interested in entering this exciting research area.
Uniqueness of air–water and liquid–liquid interfaces
The air–water and liquid–liquid interfaces are flat spaces between air and water, and two immiscible liquid phases, respectively, which can provide 2D-confined polymerization for the monomers but do not restrict their facile mobility. Notably, the interfacial roughness, and the monomer orientation and interaction at the interfaces are key parameters and can be varied to tune the migration of monomers and the equilibria, as well as the rate of chemical reactions.28 For instance, the air–water interface has been measured to be smooth with a root-mean-square roughness of about 3Å.28 The adsorption of amphiphilic molecules such as surfactants and short/medium-chain alcohols, acids, alkalis, and quaternary salts can finely tune the roughness from dozens of angstroms to nanometers.29 As a result, the varied interfacial roughness will strongly influence the mass transfer of monomers at the interfaces, which controls the reaction rate. In addition, the geometrical arrangement of monomers is of significant importance, because steric requirements for chemical reactions at interfaces may lead to strong reactivity dependence on molecular orientation for forming 2D ordered networks. In this case, the molecular orientation could be regulated by the solvent/monomer or monomer/monomer intermolecular forces, generally including electrostatic force, hydrogen-bonding, hydrophilic solvation, and hydrophobic effect.20,23,30 The high reaction reactivity and yield have been demonstrated for a variety of reactions at the interfaces in comparison to the reactions occurred in homogeneous media. Breslow et al. reported the utilization of hydrocarbon-water interface for the Diels–Alder reactions and found that the reaction rate was dramatically enhanced in comparison to that in bulk solution, due to the enforced hydrophobic interactions and stabilization of the activated complex by hydrogen-bonding at the interface.30 These above features enable the interfaces as templates to confine and direct the monomers in a 2D geometry for the bottom-up synthesis of 2D materials with controlled morphologies, chemical, and physical properties.
Development of liquid-interface-assisted synthesis strategies
The air–water interface has been employed to confine water-insoluble monomers or even nanoparticles (Fig. 1a), which can freely rotate and move around in lateral directions unless they are packed to form monolayers. Generally, a controlled amount of monomer I or ligand (crosses in Fig. 1a) in an organic solvent is spread on an aqueous solution of the monomer II or salts (dots in Fig. 1a). The evaporation of the organic solvent leaves the monomer I on the aqueous surface, and the polymerization reaction processes at the air–water interface. In this strategy, special designs are generally required for the employed monomers/precursors: (1) functionalization with hydrophilic groups ensures their sufficient spreading on the water surface, in which the hydrophilic groups are bound with the water molecules while the relatively hydrophobic parts face toward the air;23,31,32 (2) reactive units in the monomers/precursors should stack closely along the water surface, which allows the further 2D polymerization.
The Langmuir–Blodgett (LB) method also provides the air–water interface and is more convenient to synthesize densely packed monolayers with control over the stacking density of monomers by tailoring the surface pressure of water. Thereby, this method provides the opportunity to control the composition and the structure of 2D materials by non-covalent and covalent bonds at the molecular level. A general procedure can be described as follows (Fig. 1b): (1) a sub-monolayer of the first water-insoluble monomer is spread over the water surface in a LB trough; (2) after the close packing of the monomers into a dense film with highly ordered internal structure upon compression, a solution of the second monomer or metal salts is injected into the water phase; (3) with the diffusion of the second water-soluble monomer or metal salts from the bulk phase to the interface, 2D polymerization is triggered, resulting in targeted single-layers with a large area (cm2). When one repeats the transfer process, layer-stacked 2D materials can be prepared.21,24 Currently, this LB strategy has been successfully utilized for the synthesis of single-layer and multi-layer 2DCPs by us and other groups.20,21,23,24,25,31,32,33
Besides the air–water interface, liquid–liquid interface system has also been explored for the synthesis of 2DCPs. The region of liquid–liquid interface is not a layer that is one molecule thick, but usually has a thickness of a few nanometers. Thus, the liquid–liquid interface can act as a suitable 2D space to confine monomers and their polymerization to establish multi-layer structures (Fig. 1c).34,35,36,37 The selection of solvents will depend on the chosen monomers to offer them good solubility in either upper or lower phase, such as oil-water (chloroform-water, carbon tetrachloride-water, toluene-water, and n-hexane-water) and oil–oil (acetonitrile-dimethylformamide, methanol-hexane, and dimethylsulfoxide-hexane) interfaces.
Covalent chemistry methodologies for interfacial synthesis
Despite organic chemistry could offer a huge library of building blocks that can be synthesized and linkages to connect these building blocks, the current construction of 2DCPs at the air–water and liquid–liquid interfaces has been limited to only a few chemistry methodologies.
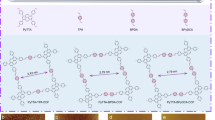
First of all, strong covalent bonds are highly appealing due to their ability to maintain the overall structural integrity of polymers. By far, two classes of irreversible covalent reactions have been employed for the interfacial synthesis of covalent-organic 2DCPs (2D polymers/2D COFs) via C–C bonds, including photo-dimerization (Fig. 2a)23,32 and Glaser coupling (Fig. 2b).26 In 2014, Schlüter and King et al. synthesized freestanding, single-layer 2DCPs at the air/water interface through photo-dimerization of anthracene-based amphiphilic monomers (A1).32 The synthesis procedure typically comprised a preorganization of photo-active monomers on water surface into a closely packed film and then the formation of covalent monolayers was triggered by the photoinduced polymerization. Subsequently, these authors employed the above strategy on antrip monomers (A2) and prepared another single-layer 2DCP with the lateral size over 1 cm2 (Fig. 3a) and the thickness of ~1.2 nm confirmed by atomic force microscopy (AFM), corresponding to the expected height of one antrip monomer.23 The molecular-level resolved image of the covalent 2DCP obtained at the water surface was elucidated by scanning tunneling microscopy (STM), indicative of periodic hexagonal structures with ultrahigh pore density (Fig. 3a). One can see that the combination of interfaces and photo-dimerization could lead to the successful synthesis of C–C-based 2DCPs. Another C–C-based 2DCP was reported by Nishihara et al. in 2017, who synthesized graphdiyne nanosheets based on hexaethynylbenzene (B1) via Glaser coupling with copper(II) acetate and pyridine as catalyst at the argon/H2O interface (Fig. 3b).26 The obtained graphdiyne nanosheets exhibited regular hexagonal shape (Fig. 3b) and a narrow distribution of thickness of ~3 nm and lateral size of ~1.5 μm. Two-dimensional grazing-incidence wide-angle X-ray scattering (2D GIWAXS) and electronic diffraction were carried out to confirm the through crystallinity of the few-layer graphdiyne, which was featured with an ABC-type stacking and a hexagonal lattice with in-plane and out-of-plane periodicities of a = b = 0.96 nm and c = 1.02 nm, respectively (Fig. 3b).
(I) 2DCPs based on covalent organic reactions and the representative monomers. a Photo-dimerization;b Glaser coupling; c cyclotrimerization reaction; d Schiff-base reaction. (II) 2DCPs based oncoordination reactions and the representative monomers. e paddle-wheel linkage; f metal-terpyridinelinkage; g metal-dipyrrin linkage; h metal-bis(diimino)/-bis(dihydroxy)/-bis(dithiolene) linkages
Representative 2DCPs based on carbon-carbon bonds by a photo-polymerization and b Glaser coupling, c imine-bonds by Schiff-base reaction, and d conjugated planar metal-bis(dithiolene) linkages. Reproduced with permission from ref, 23 ref, 26 ref 25 and ref, 24 respectively. Copyrights 2015 American Chemical Society, 2017 American Chemical Society, 2016 Nature Publishing Group and 2015 John Wiley & Sons, Inc, respectively
Besides the irreversible reactions, thermodynamically reversible covalent bonds like triazine (Fig. 2c) and imine (Fig. 2d), which can provide error correction for the synthesis of highly ordered structures, have been employed for the synthesis of 2DCPs at the air–water and liquid–liquid interfaces. For instance, Xu et al. demonstrated the one-pot synthesis of single-layer and few-layer triazine-based 2DCPs through cyclotrimerization reaction of 1,4-dicyanobenzene (C1) at the CH2Cl2–CF3SO3H interface in 2017.38 As is known, cyclotrimerization polymerization of nitriles has been early developed by Thomas and Antonietti for the synthesis of triazine-based bulk COFs in the presence of Lewis acid at high temperature.39 Here, the obtained 2DCPs were featured with the lateral size ranging from micrometers to several hundred micrometers and the thickness of 0.9 (monolayer)-3 (multilayer) nm, and exhibited promising semiconducting property with a high on/off ratio of 103 and a remarkable mobility of 0.15 cm2 V−1 s−1. Recently, our group achieved wafer-sized 2DCP monolayer (~0.7 nm in thickness) based on Schiff-base polycondensation of 2,5-dihydroxyterephthalaldehyde (D1) and 5,10,15,20-tetrakis(4-aminophenyl)21H,23H-porphyrin-Co(II) (D2) at the air–water interface (Fig. 3c).25 The resultant 2DCP displayed an outstanding Young’s modulus (267 ± 30 GPa) comparable to that of graphene, and presented multi-functions such as an organic semiconducting layer for thin film transistor and an active catalyst for electrocatalytic hydrogen generation from water. A graphene (G) encapsulation method to enhance the electron irradiation resistance of a monolayer by fabricating the G/2DP/G sandwich structure was established (Fig. 3c), offering clear electron diffraction proof for the long-range order in this single-layer 2DCP. This work opens the door for interfacial synthesis of 2DCPs based on Schiff-base reaction even in aqueous system. Intriguing work by Zhang,33 Banerjee,36 and Dichtel37 also confirmed the power of the liquid-interface-assisted synthesis by developing a series of imine-based 2D covalent-organic films with various skeletons and tunable thickness.
Coordination chemistry methodologies for interfacial synthesis
Apart from the covalent chemistry, coordination chemistry has also been explored for the synthesis of single-layer and multi-layer metal-organic 2DCPs (2D MOFs) at the air–water and liquid–liquid interfaces by Kitagawa,20 Schlüter,21 Nishihara,22,35 Zhu,34 Marinescu,40 Louie,41 Xu42, and our group.24,43 Thus far, two classes of 2D MOFs have been reported via the liquid-interface-assisted synthesis, i.e., none-conjugated and fully conjugated ones, which are distinguished by their structural planarity and charge delocalization degree. One early representative work was reported by Talham et al. in 2002,31 who synthesized a crystalline iron-nickel cyanide-bridged network by polymerization of amphiphilic pentacyanoferrate(III) complexes and iron ions at the air/water interface in a LB trough. Following this LB method, Kitagawa et al. reported the synthesis of a monolayer 2D MOF comprising [5,10,15,20-tetrakis(4-carboxyphenyl)porphyrin]cobalt(II) (E1) ligands and paddle-wheel linkages (Fig. 2e) with two Cu2+ and four COO− groups in 2010.20 Subsequently, some photoactive 2D MOF films have been prepared based on terpyridine (F1, F2)21,44 and dipyrrin (G1)35 ligands by the liquid-interface-assisted synthesis and exhibited potential photoelectric conversion ability. However, such 2D MOFs behaved low conductivity (<10−10 S/cm) because of their low conjugation, localized molecular orbitals, and minimal band dispersion in their intrinsic electronic structures, thus hampering their application in electronics. On the other hand, fully conjugated 2D MOFs have been emerging with the realization of high electrical conductivity (10−3~103 S/cm) since the report about Cu/Ni-catecholates (H4) by Yaghi et al. in 2012.45 Currently, fully conjugated 2D MOFs have been synthesized by linking N, O, or S ortho-disubstituted benzene (H1-H3)22,41,46 or triphenylene (H4-H6)24,40,42 ligands with square planar linkages, such as metal-bis(diimino)/-bis(dihydroxy)/bis(dithiolene), as well as their hybrids (Fig. 2h), and are featured with hexagonal lattices, layered structures and full π–d conjugation in 2D planes. For instance, in 2013,22 Nishihara et al. employed the CH2Cl2/H2O interface to prepare multi-layer 2D MOFs comprising benzene-fused nickel-bis(dithiolene) complexes (H2-Ni) with a lateral size of ∼100 μm and a thickness of 1–2 μm, which could exhibit conductivity as high as 1.6 × 102 S cm−1. After that, Zhu et al. synthesized H2-Cu 2D MOF films at the CH2Cl2/H2O interface and achieved much higher conductivity of 1.58 × 103 S cm−1.34 In 2015, our group demonstrated the synthesis of a large area, single-layer (~0.7 nm in thickness), fully conjugated H5-Ni 2D MOF by LB method (Fig. 3d).24 The crystallinity of the monolayer was confirmed by cryogenic transmission electron microscopy (cryo-TEM) at -175 °C, which provided a typical hexagonal electron diffraction pattern (Fig. 3d). Benefiting from their electrically conducting feature and the incorporation of highly active catalytic sites, such 2D MOF films served as carbon-rich electrode materials for electrocatalytic water splitting. By far, the conducting, conjugated 2D MOFs have been explored for wide applications, such as field effect transistor,42 chemiresistive sensing,47 and energy storage and conversion.24,40,43,48,49,50
Challenges and opportunities
In this Perspective article, the liquid-interface-assisted synthesis is highlighted for the preparation of covalent-organic and metal-organic 2DCPs. The controlled synthesis of single-layer or multi-layer 2DCPs with fine-tuned lateral size and thickness have been explored. Despite of the recent success in this exciting research area, many critical issues remain to be addressed.
-
1.
The first issue concerns the synthesis of 2DCPs with the control of their crystalline domains, grain boundaries and edge structures. In this case, the liquid-interface-assisted synthesis relies on the physical and chemical properties of the precursors such as solubility, thermal stability, sensitivity, catalytic activity, and functional groups, as well as special interactions with interfaces. Additionally, fundamental understanding about the interfacial synthesis remains far from mature, such as the control of the preorganization of precursors at the interfaces, the (polymerization) reaction efficiency at interfaces, the structural and morphological control at interfaces by fine-tuning external conditions like concentration, ratio, pH, catalyst, and the defect control and correction, etc.
-
2.
Compared with the on-surface synthesis and the solution exfoliation approaches, the liquid-interface assisted synthesis method also has intrinsic limitation. For instance, the reaction temperatures cannot be varied over a large range. Generally, the liquid–liquid interfaces are allowed for reaction at relatively wide temperature range (~5 °C to ~100 °C)38 while the air–water interface is limited from ~5 °C to ~50 °C to maintain the interfaces.23,24,25 In this case, only a few irreversible covalent reactions (photopolymerization and Glaser coupling) and dynamic covalent reactions (cyclotrimerization of nitriles and Schiff-base reaction) were explored for synthesizing covalent-organic 2DCPs while other linkage methodologies remain to be addressed. On the other hand, interfacial synthesis of metal-organic 2DCPs was also limited to several coordination models. Thus, future boosting growth in the development of rich chemistry methodologies for interfacial synthesis is expected to enrich the family of 2DCPs.
-
3.
Apart from the air–water and liquid–liquid interfaces, as well as the mentioned on-surface synthesis (also regarded as vapor-solid or vacuum/solid interfaces), interfaces also involve many other potential candidates that can be employed for the synthesis of 2DCPs, such as salt or ice surfaces, other 2DM surfaces (like graphene and metal dichalcogenides), and the outside/inside surfaces of hard and/or soft layered structures (like clays, layered double hydroxides, and surfactant mono-layers and bi-layers). These interfaces are also expected to act as templates, which allow the concentration and the ordered pre-organization of the monomers along/within the 2D-confined space via non-covalent interactions, including hydrogen bonding, electrostatic interactions, hydrophobic force, and π–π stacking, to hold the reactive sites close with each other, finally leading to a template-direct 2D polymerization. Till now, the corresponding researches are still under development, which are able to enrich the interface-assisted synthesis strategies toward multi-diverse 2DCPs.
-
4.
The transfer processing of these 2DMs from interfaces should also be very careful due to low mechanical strength resulting in structural distortion and folding and tearing of the sheets, and possible oxidation for some air-sensitive 2DCPs in ambient conditions leading to the structural decomposition. These issues are highly related to the future development of interfacial synthesis strategies and processing technologies.
-
5.
The structural characterization at the molecular or atomic level is a key challenge for the interfaces, which is of critical importance for understanding the crystal growth mechanism. In recent years, some in-situ characterization techniques have been developed, such as in-situ TEM, XRD, and Raman spectroscopy. How to efficiently apply these techniques for probing the interfacial growth of 2DCPs is not easy. Particularly, the structural confirmation of these single-layers by high resolution HRTEM at the atomic scale remains challenging due to their high sensitivity to the electron irradiation of TEM. Nevertheless, many imaging technologies are under exploration to reduce the structural damage, such as imaging under a suitable accelerating voltage,45 at low electron beam dose or at extra low temperature,16,24 and the developed graphene (G) encapsulation method.25 In addition, the STM imaging of the single layers transferred from air–water or liquid–liquid interfaces onto solid substrates has not been fully satisfied due to the possible introduction of contaminations, and folding and tearing of the monolayers during the transfer progress.22,23
-
6.
Finally, the applications of 2DCPs obtained by the liquid-interface-assisted synthesis remain at the infancy stage. One can expect that covalent-organic and metal-organic 2DCPs with large area, highly ordered opened-porous structures, ultra-thin and free-standing feature, and integration of abundant functional units will open up a wide range of applications in optics,35 electronics,22,25,34,38,41,42,45 sensing,44,47 separation,18,37 energy storage, and conversion.24,40,43,46,48,49,50 Nevertheless, the superior performances in these applications highly rely on structural control of the 2DCPs at the atomic and molecular level. Thus, the reliable and fundamental structure-property relationship should be addressed, which will flourish the development of 2DCPs via liquid-interface assisted synthesis in the future.
References
Sakamoto, J., van Heijst, J., Lukin, O. & Schlüter, A. D. Two-dimensional polymers: just a dream of synthetic chemists? Angew. Chem. Int. Ed. 48, 1030–1069 (2009).
Payamyar, P., King, B. T., Ottinger, H. C. & Schlüter, A. D. Two-dimensional polymers: concepts and perspectives. Chem. Commun. 52, 18–34 (2016).
Colson, J. W. & Dichtel, W. R. Rationally synthesized two-dimensional polymers. Nat. Chem. 5, 453–465 (2013).
Jin, Y., Hu, Y. Y. & Zhang, W. Tessellated multiporous two-dimensional covalent organic frameworks. Nat. Rev. Chem. 1, 0056 (2017).
Huang, N., Wang, P. & Jiang, D. Covalent organic frameworks: a materials platform for structural and functional designs. Nat. Rev. Mater. 1, 16068 (2016).
Diercks, C. S. & Yaghi, O. M. The atom, the molecule, and the covalent organic framework. Science 355, eaal1585 (2017).
Dong, R., Zhang, T. & Feng, X. Interface-assisted synthesis of 2D materials: trend and challenges. Chem. Rev. https://doi.org/10.1021/acs.chemrev.8b00056 (2018).
Zhao, M., Lu, Q., Ma, Q. & Zhang, H. Two-dimensional metal-organic framework nanosheets. Small Methods 1, 1600030 (2017). n/a.
Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004).
Grill, L. et al. Nano-architectures by covalent assembly of molecular building blocks. Nat. Nanotech. 2, 687–691 (2007).
Bieri, M. et al. Porous graphenes: two-dimensional polymer synthesis with atomic precision. Chem. Commun. 45, 6919–6921 (2009).
Dienstmaier, J. F. et al. Isoreticular two-dimensional covalent organic frameworks synthesized by on-surface condensation of diboronic acids. ACS Nano 6, 7234–7242 (2012).
Liu, X.-H. et al. On-surface synthesis of single-layered two-dimensional covalent organic frameworks via solid-vapor interface reactions. J. Am. Chem. Soc. 135, 10470–10474 (2013).
Cardenas, L. et al. Synthesis and electronic structure of a two dimensional π-conjugated polythiophene. Chem. Sci. 4, 3263–3268 (2013).
Urgel, J. I. et al. Orthogonal insertion of lanthanide and transition-metal atoms in metal-organic networks on surfaces. Angew. Chem. Int. Ed. 54, 6163–6167 (2015).
Kissel, P. et al. A two-dimensional polymer prepared by organic synthesis. Nat. Chem. 4, 287 (2012).
Bunck, D. N. & Dichtel, W. R. Bulk synthesis of exfoliated two-dimensional polymers using hydrazone-linked covalent organic frameworks. J. Am. Chem. Soc. 135, 14952–14955 (2013).
Peng, Y. et al. Metal-organic framework nanosheets as building blocks for molecular sieving membranes. Science 346, 1356–1359 (2014).
Liu, W. et al. A two-dimensional conjugated aromatic polymer via C-C coupling reaction. Nat. Chem. 9, 563–570 (2017).
Makiura, R. et al. Surface nano-architecture of a metal-organic framework. Nat. Mater. 9, 565–571 (2010).
Bauer, T. et al. Synthesis of free-standing, monolayered organometallic sheets at the air/water interface. Angew. Chem. Int. Ed. 50, 7879–7884 (2011).
Kambe, T. et al. π-Conjugated nickel bis(dithiolene) complex nanosheet. J. Am. Chem. Soc. 135, 2462–2465 (2013).
Murray, D. J. et al. Large area synthesis of a nanoporous two-dimensional polymer at the air/water interface. J. Am. Chem. Soc. 137, 3450–3453 (2015).
Dong, R. et al. Large-area, free-standing, two-dimensional supramolecular polymer single-layer sheets for highly efficient electrocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 54, 12058–12063 (2015).
Sahabudeen, H. et al. Wafer-sized multifunctional polyimine-based two-dimensional conjugated polymers with high mechanical stiffness. Nat. Commun. 7, 13461 (2016).
Matsuoka, R. et al. Crystalline graphdiyne nanosheets produced at a gas/liquid or liquid/liquid interface. J. Am. Chem. Soc. 139, 3145–3152 (2017).
Stassen, I. et al. An updated roadmap for the integration of metal-organic frameworks with electronic devices and chemical sensors. Chem. Soc. Rev. 46, 3185–3241 (2017).
Braslau, A. et al. Surface roughness of water measured by X-ray reflectivity. Phys. Rev. Lett. 54, 114–117 (1985).
Dong, R. & Hao, J. Complex fluids of poly(oxyethylene) monoalkyl ether nonionic surfactants. Chem. Rev. 110, 4978–5022 (2010).
Rideout, D. C. & Breslow, R. Hydrophobic acceleration of Diels-Alder reactions. J. Am. Chem. Soc. 102, 7816–7817 (1980).
Culp, J. T. et al. Supramolecular assembly at interfaces: formation of an extended two-dimensional coordinate covalent square grid network at the air-water interface. J. Am. Chem. Soc. 124, 10083–10090 (2002).
Payamyar, P. et al. Synthesis of a covalent monolayer sheet by photochemical anthracene dimerization at the air/water interface and its mechanical characterization by AFM indentation. Adv. Mater. 26, 2052–2058 (2014).
Dai, W. et al. Synthesis of a two-dimensional covalent organic monolayer through dynamic imine chemistry at the air/water iunterface. Angew. Chem. Int. Ed. 55, 213–217 (2016).
Huang, X. et al. A two-dimensional π-d conjugated coordination polymer with extremely high electrical conductivity and ambipolar transport behaviour. Nat. Commun. 6, 7408 (2015).
Sakamoto, R. et al. A photofunctional bottom-up bis(dipyrrinato)zinc(II) complex nanosheet. Nat. Commun. 6, 6713 (2015).
Dey, K. et al. Selective molecular separation by interfacially crystallized covalent organic framework thin films. J. Am. Chem. Soc. 139, 13083–13091 (2017).
Matsumoto, M. et al. Lewis-acid-catalyzed interfacial polymerization of covalent organic framework films. Chem 4, 308–317 (2018).
Liu, J. et al. Solution synthesis of semiconducting two-dimensional polymer via trimerization of carbonitrile. J. Am. Chem. Soc. 139, 11666–11669 (2017).
Algara-Siller, G. et al. Triazine-based graphitic carbon nitride: a two-dimensional semiconductor. Angew. Chem. Int. Ed. 53, 7450–7455 (2014).
Clough, A. J., Yoo, J. W., Mecklenburg, M. H. & Marinescu, S. C. Two-dimensional metal-organic surfaces for efficient hydrogen evolution from water. J. Am. Chem. Soc. 137, 118–121 (2015).
Lahiri, N. et al. Hexaaminobenzene as a building block for a family of 2D coordination polymers. J. Am. Chem. Soc. 139, 19–22 (2017).
Wu, G. et al. Porous field-effect transistors based on a semiconductive metal-organic framework. J. Am. Chem. Soc. 139, 1360–1363 (2017).
Dong, R. et al. Immobilizing molecular metal dithiolene-diamine complexes on 2D metal-organic frameworks for electrocatalytic H2 Production. Chem. Eur. J. 23, 2255–2260 (2017).
Takada, K. et al. An electrochromic bis(terpyridine)metal complex nanosheet. J. Am. Chem. Soc. 137, 4681–4689 (2015).
Hmadeh, M. et al. New porous crystals of extended metal-catecholates. Chem. Mater. 24, 3511–3513 (2012).
Sun, X. et al. Bis(aminothiolato)nickel nanosheet as a redox switch for conductivity and an electrocatalyst for the hydrogen evolution reaction. Chem. Sci. 8, 8078–8085 (2017).
Campbell, M. G. et al. Cu3(hexaiminotriphenylene)2: an electrically conductive 2D metal-organic framework for chemiresistive sensing. Angew. Chem. Int. Ed. 54, 4349–4352 (2015).
Miner, E. M. et al. Electrochemical oxygen reduction catalysed by Ni3(hexaiminotriphenylene)2. Nat. Commun. 7, 10942 (2016).
Sheberla, D. et al. Conductive MOF electrodes for stable supercapacitors with high areal capacitance. Nat. Mater. 16, 220–224 (2016).
Feng, D. et al. Robust and conductive two-dimensional metal-organic frameworks with exceptionally high volumetric and areal capacitance. Nat. Energy 3, 30–36 (2018).
Acknowledgements
This work was financially supported from ERC Grant on 2DMATER, UP-GREEN, and EU Graphene Flagship, COORNET (SPP 1928), as well as the German Science Council, Center of Advancing Electronics Dresden, EXC1056, (cfaed) and OR 349/1. R.D. appreciates the great support from Prof. Xinliang Feng. R.D. also thanks the helpful discussion with Prof. Zhikun Zheng. R.D. also acknowledges his team members in the research of interfacial synthesis of 2D covalent polymers and 2D MOFs, particularly Dr. Tao Zhang, Dr. Lihuan Wang, Dr. Zhong Haixia, Miss Chongqing Yang, Mr. Hafeesudeen Sahabudeen, Mr. Kejun Liu, Mr. SangWook Park, Mr. Mingchao Wang, and Mr. Zhiyong Wang.
Author information
Authors and Affiliations
Contributions
R.H.D. conceived the project and shaped the concept. L.H.W. researched the literature and constructed the Perspective structure. H.S. and T.Z. contributed to the figures. All the authors participated in discussing. R.H.D. and L.H.W. co-wrote the manuscript with contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, L., Sahabudeen, H., Zhang, T. et al. Liquid-interface-assisted synthesis of covalent-organic and metal-organic two-dimensional crystalline polymers. npj 2D Mater Appl 2, 26 (2018). https://doi.org/10.1038/s41699-018-0071-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41699-018-0071-5
This article is cited by
-
On-liquid-gallium surface synthesis of ultrasmooth thin films of conductive metal–organic frameworks
Nature Synthesis (2024)
-
Mechanistic insights into the deformation and degradation of a 2D metal organic framework
npj 2D Materials and Applications (2023)
-
Metal-organic layers: Preparation and applications
Science China Materials (2023)
-
Two-dimensional Metal-organic Frameworks and Derivatives for Electrocatalysis
Chemical Research in Chinese Universities (2020)