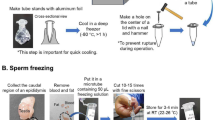
Abstract
Laboratory rats have been used in biomedical research for over 170 years. Recently, genome editing technology has facilitated the generation of genetically modified rats worldwide. This development has increased the demand for efficient preservation and production of rat resources. Sperm cryopreservation is the most efficient and robust means to archive genetic resources, and this technique reduces the number of animals required for colony management. Previously, we have reported a protocol for rat sperm cryopreservation and in vitro fertilization using frozen–thawed sperm. Here we describe an improved in vitro fertilization protocol to enhance the fertilization rate of cryopreserved sperm in major strains of rats. In this optimized protocol, treatment of frozen–thawed rat sperm with a high concentration of bovine serum albumin (40 mg/ml) results in a high in vitro fertilization rate. This protocol consists of three main steps: preparation of cryopreserved sperm, in vitro fertilization using cryopreserved sperm and embryo transfer. This process takes approximately 1 month to produce live pups from cryopreserved sperm. This protocol can be easily implemented by researchers and technicians with experience in reproductive engineering technology; it can also be used, albeit with some practice, by researchers and technicians who have no experience in reproductive techniques. This sperm cryopreservation and in vitro fertilization protocol for rats will provide an efficient system for the archiving and production of genetically modified rats for the transgenic community.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
Data availability
The data supporting the study findings are available within the paper and its supplementary information files.
Change history
21 October 2022
In the version of this article initially published, the label “EGFP” now appearing on the second from bottom row of Table 4 was originally placed on the third from bottom row. The table has been amended in the HTML and PDF versions of the article.
References
Jacob, H. J. Functional genomics and rat models. Genome Res. 9, 1013–1016 (1999).
Suckow, M. A., Hankenson, F. C. & Wilson, R. P. The Laboratory Rat 3rd edn (Academic Press, 2020).
Li, D. et al. Heritable gene targeting in the mouse and rat using a CRISPR–Cas system. Nat. Biotechnol. 31, 681–683 (2013).
Li, W., Teng, F., Li, T. & Zhou, Q. Simultaneous generation and germline transmission of multiple gene mutations in rat using CRISPR–Cas systems. Nat. Biotechnol. 31, 684–686 (2013).
Yoshimi, K. et al. ssODN-mediated knock-in with CRISPR–Cas for large genomic regions in zygotes. Nat. Commun. 7, 10431 (2016).
Neff, E. P. Rats on the rise. Lab. Anim. https://doi.org/10.1038/s41684-021-00812-0 (2021).
Aitman, T. J. et al. Progress and prospects in rat genetics: a community view. Nat. Genet. 40, 516–522 (2008).
Serikawa, T. et al. National BioResource Project-Rat and related activities. Exp. Anim. 58, 333–341 (2009).
Hart-Johnson, S. & Mankelow, K. Archiving genetically altered animals: a review of cryopreservation and recovery methods for genome edited animals. Lab. Anim. https://doi.org/10.1177/00236772211007306 (2021).
Marschall, S. & Hrabé de Angelis, M. Cryopreservation of mouse spermatozoa: double your mouse space. Trends Genet. 15, 128–131 (1999).
Agca, Y. & Agca, C. Cryopreservation of mouse sperm for genome banking. Methods Mol. Biol. 2180, 401–412 (2021).
Chulavatnatol, M. Motility initiation of quiescent spermatozoa from rat caudal epididymis: effects of pH, viscosity, osmolality and inhibitors. Int. J. Androl. 5, 425–436 (1982).
Si, W., Benson, J. D., Men, H. & Critser, J. K. Osmotic tolerance limits and effects of cryoprotectants on the motility, plasma membrane integrity and acrosomal integrity of rat sperm. Cryobiology 53, 336–348 (2006).
Kim, S., Agca, C. & Agca, Y. Changes in rat spermatozoa function after cooling, cryopreservation and centrifugation processes. Cryobiology 65, 215–223 (2012).
Nakatsukasa, E., Inomata, T., Ikeda, T., Shino, M. & Kashiwazaki, N. Generation of live rat offspring by intrauterine insemination with epididymal spermatozoa cryopreserved at −196 degrees C. Reproduction 122, 463–467 (2001).
Seita, Y., Sugio, S., Ito, J. & Kashiwazaki, N. Generation of live rats produced by in vitro fertilization using cryopreserved spermatozoa. Biol. Reprod. 80, 503–510 (2009).
Yamashiro, H. et al. Extracellular ATP and dibutyryl cAMP enhance the freezability of rat epididymal sperm. J. Am. Assoc. Lab. Anim. Sci. 49, 167–172 (2010).
Nakagata, N., Mikoda, N., Nakao, S., Nakatsukasa, E. & Takeo, T. Establishment of sperm cryopreservation and in vitro fertilisation protocols for rats. Sci. Rep. 10, 93 (2020).
Jiang, J. Y. & Tsang, B. K. Optimal conditions for successful in vitro fertilization and subsequent embryonic development in Sprague–Dawley rats. Biol. Reprod. 71, 1974–1979 (2004).
Popova, E., Bader, M. & Krivokharchenko, A. Strain differences in superovulatory response, embryo development and efficiency of transgenic rat production. Transgenic Res. 14, 729–738 (2005).
Davis, B. K. Influence of serum albumin on the fertilizing ability in vitro of rat spermatozoa. Proc. Soc. Exp. Biol. Med. 151, 240–243 (1976).
Visconti, P. E. et al. Cholesterol efflux-mediated signal transduction in mammalian sperm. beta-cyclodextrins initiate transmembrane signaling leading to an increase in protein tyrosine phosphorylation and capacitation. J. Biol. Chem. 274, 3235–3242 (1999).
Takeo, T. et al. Methyl-β-cyclodextrin improves fertilizing ability of C57BL/6 mouse sperm after freezing and thawing by facilitating cholesterol efflux from the cells. Biol. Reprod. 78, 546–551 (2008).
Kikuchi, K. et al. Reproduction in pigs using frozen–thawed spermatozoa from epididymis stored at 4 C. J. Reprod. Dev. 45, 345–350 (1999).
Nakata, M. et al. Successful production of offspring using cryopreserved sperm via nonsurgical artificial insemination in rats. J. Reprod. Dev. 58, 501–504 (2012).
Quinn, P., Kerin, J. F. & Warnes, G. M. Improved pregnancy rate in human in vitro fertilization with the use of a medium based on the composition of human tubal fluid. Fertil. Steril. 44, 493–498 (1985).
Quinn, P., Warnes, G. M., Kerin, J. F. & Kirby, C. Culture factors affecting the success rate of in vitro fertilization and embryo transfer. Ann. N. Y. Acad. Sci. 442, 195–204 (1985).
Takeo, T. & Nakagata, N. Cryobanking and recovery of genetically modified mice. Methods Mol. Biol. 2066, 195–209 (2020).
Takeo, T., Sztein, J. & Nakagata, N. The CARD method for mouse sperm cryopreservation and in vitro fertilization using frozen–thawed sperm. Methods Mol. Biol. 1874, 243–256 (2019).
Nakagata, N. [Cryopreservation of unfertilized rat oocytes by ultrarapid freezing]. Jikken Dobutsu 41, 443–447 (1992).
Aoto, T., Takahashi, R. & Ueda, M. A protocol for rat in vitro fertilization during conventional laboratory working hours. Transgenic Res. 20, 1245–1252 (2011).
Honda, A. et al. Efficient derivation of knock-out and knock-in rats using embryos obtained by in vitro fertilization. Sci. Rep. 9, 11571 (2019).
Kito, S. et al. Improved in vitro fertilization and development by use of modified human tubal fluid and applicability of pronucleate embryos for cryopreservation by rapid freezing in inbred mice. Comp. Med. 54, 564–570 (2004).
Biggers, J. D., Whitten, W. K., Whittingham, D. G. The Culture of Mouse Embryos In Vitro pp. 86–116 (San Francisco: W. H. Freeman & Co., 1971).
Miyamoto, H. & Chang, M. C. In vitro fertilization of rat eggs. Nature 241, 50–52 (1973).
Niwa, K., Imai, H., Kim, C. I. & Iritani, A. Fertilization in vitro of hamster and mouse eggs in a chemically defined medium. J. Reprod. Fertil. 58, 109–114 (1980).
Toyoda, Y. & Chang, M. C. Capacitation of epididymal spermatozoa in a medium with high K–Na ratio and cyclic AMP for the fertilization of rat eggs in vitro. J. Reprod. Fertil. 36, 125–134 (1974).
Toyoda, Y. & Chang, M. C. Fertilization of rat eggs in vitro by epididymal spermatozoa and the development of eggs following transfer. J. Reprod. Fertil. 36, 9–22 (1974).
Miyoshi, K., Tanaka, N. & Niwa, K. Penetration in vitro of naturally ovulated rat eggs and the development of eggs in a chemically defined medium. J. Mamm. Ova Res. 12, 35–39 (1995).
Miyoshi, K., Abeydeera, L. R., Okuda, K. & Niwa, K. Effects of osmolarity and amino acids in a chemically defined medium on development of rat one-cell embryos. J. Reprod. Fertil. 103, 27–32 (1995).
Oh, S. H., Miyoshi, K. & Funahashi, H. Rat oocytes fertilized in modified rat 1-cell embryo culture medium containing a high sodium chloride concentration and bovine serum albumin maintain developmental ability to the blastocyst stage. Biol. Reprod. 59, 884–889 (1998).
Hirabayash, M. et al. Offspring derived from intracytoplasmic injection of transgenic rat sperm. Transgenic Res. 11, 221–228 (2002).
Kaneko, T., Kimura, S. & Nakagata, N. Offspring derived from oocytes injected with rat sperm, frozen or freeze-dried without cryoprotection. Theriogenology 68, 1017–1021 (2007).
Seita, Y., Ito, J. & Kashiwazaki, N. Removal of acrosomal membrane from sperm head improves development of rat zygotes derived from intracytoplasmic sperm injection. J. Reprod. Dev. 55, 475–479 (2009).
Said, S., Han, M. S. & Niwa, K. Development of rat oocytes following intracytoplasmic injection of sperm heads isolated from testicular and epididymal spermatozoa. Theriogenology 60, 359–369 (2003).
Whittingham, D. G. Survival of rat embryos after freezing and thawing. J. Reprod. Fertil. 43, 575–578 (1975).
Kono, T., Suzuki, O. & Tsunoda, Y. Cryopreservation of rat blastocysts by vitrification. Cryobiology 25, 170–173 (1988).
Anzai, M. et al. [Cryopreservation of in vitro fertilized embryos from transgenic rat by ultrarapid freezing]. Jikken Dobutsu 43, 247–250 (1994).
Hirabayashi, M. & Chaya, N. Low temperature storage of rat 2-cell embryos by vitrification. J. Mamm. Ova Res. 7, 72–77 (1990).
Tada, N., Sato, M., Mizorogi, T., Kasai, K. & Ogawa, S. Efficient cryopreservation of hairless mutant (bald) and normal Wistar rat embryos by vitrification. Lab. Anim. Sci. 45, 323–325 (1995).
Sato, M., Yokokawa, K., Kasai, K. & Tada, N. Successful vitrification of stroke-prone spontaneously hypertensive and normal Wistar rat 2-cell embryos. Lab. Anim. 30, 132–137 (1996).
Jiang, J. Y., Umezu, M. & Sato, E. Vitrification of two-cell rat embryos derived from immature hypothyroid rdw rats by in vitro fertilization in ethylene glycol-based solutions. Cryobiology 38, 160–164 (1999).
Han, M. S., Niwa, K. & Kasai, M. Vitrification of rat embryos at various developmental stages. Theriogenology 59, 1851–1863 (2003).
Hirabayashi, M., Takahashi, R., Sekiguchi, J. & Ueda, M. Viability of transgenic rat embryos after freezing and thawing. Exp. Anim. 46, 111–115 (1997).
Pfaff, R. T. et al. Cryobiology of rat embryos I: determination of zygote membrane permeability coefficients for water and cryoprotectants, their activation energies, and the development of improved cryopreservation methods. Biol. Reprod. 63, 1294–1302 (2000).
Takahashi, R., Hirabayashi, M. & Ueda, M. Production of transgenic rats using cryopreserved pronuclear-stage zygotes. Transgenic Res. 8, 397–400 (1999).
Isachenko, V. V., Isachenko, E. F., Ostashko, F. I. & Grishchenko, V. I. Ultrarapid freezing of rat embryos with rapid dilution of permeable cryoprotectants. Cryobiology 34, 157–164 (1997).
Rall, W. F. et al. Factors affecting the efficiency of embryo cryopreservation and rederivation of rat and mouse models. ILAR J. 41, 221–227 (2000).
Taketsuru, H. & Kaneko, T. Efficient collection and cryopreservation of embryos in F344 strain inbred rats. Cryobiology 67, 230–234 (2013).
Taketsuru, H. & Kaneko, T. Tolerance to vitrification of rat embryos at various developmental stages. Cryobiology 84, 1–3 (2018).
Fukuda, Y. et al. Small-volume vitrification and rapid warming yield high survivals of one-cell rat embryos in cryotubes. Biol. Reprod. https://doi.org/10.1093/biolre/ioab059 (2021).
Varisli, O., Uguz, C., Agca, C. & Agca, Y. Various physical stress factors on rat sperm motility, integrity of acrosome, and plasma membrane. J. Androl. 30, 75–86 (2009).
Takeo, T., Nakao, S., Nakagawa, Y., Sztein, J. M. & Nakagata, N. Cryopreservation of mouse resources. Lab. Anim. Res. 36, 33 (2020).
Hino, C., Ueda, J., Funakoshi, H. & Matsumoto, S. Defined oocyte collection time is critical for reproducible in vitro fertilization in rats of different strains. Theriogenology 144, 146–151 (2020).
Nakagata, N. [Embryo transfer through the wall of the fallopian tube in mice]. Jikken Dobutsu 41, 387–388 (1992).
Acknowledgements
We thank K. Uchiyama (Livestock Improvement Association of Japan, Inc.), H. Yamashiro (Graduate School of Science and Technology, Niigata University) and A. Takizawa (Human & Molecular Genetic Centre, Medical College of Wisconsin) for invaluable advice and discussions. We are grateful to our staff: T. Kondo, Y. Haruguchi, K. Yamashita, E. Ishida, M. Sakaguchi and Y. Deshimaru, for their technical support and helpful discussions. We would like to thank Enago (www.enago.jp) for English language review. This work was supported by a grant from Kyudo Company.
Author information
Authors and Affiliations
Contributions
N.N. designed the work, and N.N., N.M., S.N. and K.Y. contributed to the acquisition of the data. N.N., N.M., S.N. and T.T. wrote the initial draft of the manuscript. S.T. contributed to the illustrations of the procedures. E.N. contributed to the interpretation of the data. T.T. contributed to the analysis, interpretation of the data and revision of the manuscript. R.M. produced the video abstract. All authors approved the final version of the manuscript, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare competing financial interests. The present study was partially supported by a grant of Kyudo Co. Ltd. N.M. is a member of Kyudo Co. Ltd. K.Y. received a grant from Kyudo Co. Ltd. N.N. is a member of collaboration laboratory between Kyudo Co. Ltd and Kumamoto University. T.T., S.N., R.M., S.T. and E.N. declare no financial interests.
Peer review
Peer review information
Lab Animal thanks Elizabeth Bryda and Martha Koerner for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2, and Supplementary Fig. 1.
Supplementary Video 1
Animation abstract. The animation abstract introduces the optimized IVF protocol of cryopreserved rat sperm by combining preincubation of sperm with increased concentration of BSA and the removal of cumulus cells that surround the oocytes. This cryopreservation and IVF protocol has the potential to be implemented in several rat strains and will provide an efficient system for the archiving and production of genetically modified rats for the transgenic community.
Supplementary Video 2
Motility of frozen–thawed rat sperm after 120 min preincubation. Frozen–thawed rat sperm recovered motility during the preincubation. Sperm with good motility were observed at the edge of the drop.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Takeo, T., Nakao, S., Mikoda, N. et al. Optimized protocols for sperm cryopreservation and in vitro fertilization in the rat. Lab Anim 51, 256–274 (2022). https://doi.org/10.1038/s41684-022-01053-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41684-022-01053-5